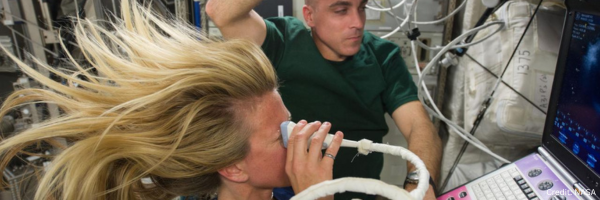
Connect with your study participants with 1-1 messaging using Appbakery apps
[vc_row][vc_column][vc_raw_html]JTNDJTIxRE9DVFlQRSUyMGh0bWwlMjBQVUJMSUMlMjAlMjItJTJGJTJGVzNDJTJGJTJGRFREJTIwWEhUTUwlMjAxLjAlMjBUcmFuc2l0aW9uYWwlMkYlMkZFTiUyMiUyMCUyMmh0dHAlM0ElMkYlMkZ3d3cudzMub3JnJTJGVFIlMkZ4aHRtbDElMkZEVEQlMkZ4aHRtbDEtdHJhbnNpdGlvbmFsLmR0ZCUyMiUzRSUwQSUzQ2h0bWwlMjB4bWxucyUzRCUyMmh0dHAlM0ElMkYlMkZ3d3cudzMub3JnJTJGMTk5OSUyRnhodG1sJTIyJTNFJTBBJTIwJTIwJTNDaGVhZCUzRSUwQSUyMCUyMCUyMCUyMCUzQ21ldGElMjBodHRwLWVxdWl2JTNEJTIyY29udGVudC10eXBlJTIyJTIwY29udGVudCUzRCUyMnRleHQlMkZodG1sJTNCJTIwY2hhcnNldCUzRFVURi04JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTNDdGl0bGUlM0VDb25uZWN0JTIwd2l0aCUyMHlvdXIlMjBzdHVkeSUyMHBhcnRpY2lwYW50cyUyMHdpdGglMjAxLTElMjBtZXNzYWdpbmclMjB1c2luZyUyMEFwcGJha2VyeSUyMGFwcHMlM0MlMkZ0aXRsZSUzRSUwQSUyMCUyMCUyMCUyMCUzQ2xpbmslMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZmb250cy5nb29nbGVhcGlzLmNvbSUyRmNzcyUzRmZhbWlseSUzRE1vbnRzZXJyYXQlMjIlMjByZWwlM0QlMjJzdHlsZXNoZWV0JTIyJTIwdHlwZSUzRCUyMnRleHQlMkZjc3MlMjIlM0UlMEElMjAlMjAlMjAlMjAlM0NsaW5rJTIwaHJlZiUzRCUyMmh0dHAlM0ElMkYlMkZmb250cy5nb29nbGVhcGlzLmNvbSUyRmNzcyUzRmZhbWlseSUzRE9wZW4lMkJTYW5zJTNBNDAwJTJDMzAwJTIyJTIwcmVsJTNEJTIyc3R5bGVzaGVldCUyMiUyMHR5cGUlM0QlMjJ0ZXh0JTJGY3NzJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTNDbGluayUyMGhyZWYlM0QlMjJkbnMtcHJlZmV0Y2glMjIlM0UlMEElMjAlMjAlMjAlMjAlM0MlMjEtLSUyMCUzQ2xpbmslMjByZWwlM0QlMjJzdHlsZXNoZWV0JTIyJTIwaHJlZiUzRCUyMnN0eWxlLmNzcyUyMiUyMCUyRiUzRSUyMC0tJTNFJTBBJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTNDc3R5bGUlMjB0eXBlJTNEJTIydGV4dCUyRmNzcyUyMiUzRSUwQSUwOSUwOS5lbWFpbENvbnRhaW5lci13aGl0ZSU3QiUwQSUwOSUwOSUwOWJhY2tncm91bmQlM0ElMjNmZmZmZmYlM0IlMEElMDklMDklN0QlMEElMDklMDlib2R5JTdCJTBBJTA5JTA5JTA5Zm9udC1mYW1pbHklM0FNb250c2VycmF0JTNCJTBBJTA5JTA5JTA5bWFyZ2luJTNBMCUzQiUwQSUwOSUwOSUwOXBhZGRpbmclM0EwJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5aW1nJTdCJTBBJTA5JTA5JTA5Ym9yZGVyJTNBMCUyMG5vbmUlM0IlMEElMDklMDklMDloZWlnaHQlM0FhdXRvJTNCJTBBJTA5JTA5JTA5bGluZS1oZWlnaHQlM0ExMDAlMjUlM0IlMEElMDklMDklMDlvdXRsaW5lJTNBbm9uZSUzQiUwQSUwOSUwOSUwOXRleHQtZGVjb3JhdGlvbiUzQW5vbmUlM0IlMEElMDklMDklMDltYXgtd2lkdGglM0ExMDAlMjUlM0IlMEElMDklMDklN0QlMEElMDklMDlhJTdCJTBBJTA5JTA5JTA5dGV4dC1kZWNvcmF0aW9uJTNBbm9uZSUzQiUwQSUwOSUwOSUwOWNvbG9yJTNBJTIzMDAwMDAwJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5YSUyMGltZyU3QiUwQSUwOSUwOSUwOWJvcmRlciUzQTAlMjBub25lJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmltYWdlRml4JTdCJTBBJTA5JTA5JTA5ZGlzcGxheSUzQWJsb2NrJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5dGFibGUlMkN0ZCU3QiUwQSUwOSUwOSUwOWJvcmRlci1jb2xsYXBzZSUzQWNvbGxhcHNlJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmJvZHlUYWJsZSU3QiUwQSUwOSUwOSUwOWhlaWdodCUzQTEwMCUyNSUyMCUyMWltcG9ydGFudCUzQiUwQSUwOSUwOSUwOW1hcmdpbiUzQTAlM0IlMEElMDklMDklMDlwYWRkaW5nJTNBMCUzQiUwQSUwOSUwOSUwOXdpZHRoJTNBMTAwJTI1JTIwJTIxaW1wb3J0YW50JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmhlYWRpbmctMiU3QiUwQSUwOSUwOSUwOWZvbnQtc2l6ZSUzQTMwcHglM0IlMEElMDklMDklMDlmb250LXdlaWdodCUzQW5vcm1hbCUzQiUwQSUwOSUwOSUwOW1hcmdpbi1ib3R0b20lM0ExMHB4JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmhlYWRpbmctMiUyMHN0cm9uZyU3QiUwQSUwOSUwOSUwOWZvbnQtd2VpZ2h0JTNBYm9sZCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5lbWFpbENvbnRhaW5lciU3QiUwQSUwOSUwOSUwOWJhY2tncm91bmQlM0ElMjNlZmVlZWQlM0IlMEElMDklMDklN0QlMEElMDklMDkuZW1haWxDb250YWluZXItY29sb3ItMiU3QiUwQSUwOSUwOSUwOWJhY2tncm91bmQlM0ElMjNlZWYwZjMlM0IlMEElMDklMDklN0QlMEElMDklMDkuZW1haWxDb250YWluZXItY29sb3ItMiUyMC5oZWFkaW5nLTIlN0IlMEElMDklMDklMDltYXJnaW4lM0EyMHB4JTIwMCUyMDEwcHglM0IlMEElMDklMDklMDlwYWRkaW5nJTNBMCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5lbWFpbENvbnRhaW5lci1jb2xvci0yJTIwaDMlN0IlMEElMDklMDklMDlmb250LXNpemUlM0ExNnB4JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmVtYWlsQ29udGFpbmVyLWNvbG9yLTIlMjBhJTdCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjMwMGFjZWQlM0IlMEElMDklMDklN0QlMEElMDklMDkuZW1haWxDb250YWluZXItY29sb3ItMiUyMHAlN0IlMEElMDklMDklMDlmb250LXNpemUlM0ExNHB4JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmVtYWlsQ29udGFpbmVyLWZvb3RlciU3QiUwQSUwOSUwOSUwOWJhY2tncm91bmQlM0ElMjNmZjkwMGUlM0IlMEElMDklMDklN0QlMEElMDklMDkuZW1haWxDb250YWluZXIubGFzdCU3QiUwQSUwOSUwOSUwOW1hcmdpbi1ib3R0b20lM0EwJTIwJTIxaW1wb3J0YW50JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmVudHJ5JTIwdGQlN0IlMEElMDklMDklMDlwYWRkaW5nLXJpZ2h0JTNBMCUyMCUyMWltcG9ydGFudCUzQiUwQSUwOSUwOSUwOXBhZGRpbmctdG9wJTNBMCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5lbnRyeTElMjB0ZCU3QiUwQSUwOSUwOSUwOXBhZGRpbmctcmlnaHQlM0E0NXB4JTIwJTIxaW1wb3J0YW50JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmVudHJ5JTIwaDMlN0IlMEElMDklMDklMDltYXJnaW4lM0ExMHB4JTIwMCUyMDNweCUzQiUwQSUwOSUwOSU3RCUwQSUwOSU0MG1lZGlhJTIwb25seSUyMHNjcmVlbiUyMGFuZCUyMCUyOG1heC13aWR0aCUzQSUyMDQ4MHB4JTI5JTdCJTBBJTA5JTA5Lm1haWwtY29udGFpbmVyJTdCJTBBJTA5JTA5JTA5ZGlzcGxheSUzQWJsb2NrJTIwJTIxaW1wb3J0YW50JTNCJTBBJTA5JTA5JTA5d2lkdGglM0ExMDAlMjUlMjAlMjFpbXBvcnRhbnQlM0IlMEElMDklMDklN0QlMEElMEElN0QlMDklNDBtZWRpYSUyMG9ubHklMjBzY3JlZW4lMjBhbmQlMjAlMjhtYXgtd2lkdGglM0ElMjA0ODBweCUyOSU3QiUwQSUwOSUwOS5jb2wyLTElN0IlMEElMDklMDklMDlkaXNwbGF5JTNBYmxvY2slMjAlMjFpbXBvcnRhbnQlM0IlMEElMDklMDklMDl3aWR0aCUzQTEwMCUyNSUyMCUyMWltcG9ydGFudCUzQiUwQSUwOSUwOSU3RCUwQSUwQSU3RCUwOSUwOSUyM2RhdGUtdG9wJTdCJTBBJTA5JTA5JTA5dGV4dC10cmFuc2Zvcm0lM0F1cHBlcmNhc2UlM0IlMEElMDklMDklMDljb2xvciUzQSUyMzc4Nzc3NyUzQiUwQSUwOSUwOSUwOWZvbnQtc2l6ZSUzQTEycHglM0IlMEElMDklMDklN0QlMEElMDklMDklMjNlbWFpbEhlYWRlciU3QiUwQSUwOSUwOSUwOWJvcmRlci1ib3R0b20lM0ExcHglMjBzb2xpZCUyMCUyM2ViZWJlYiUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5yZWFkbW9yZSU3QiUwQSUwOSUwOSUwOWJhY2tncm91bmQlM0ElMjNmZjkwMGUlM0IlMEElMDklMDklMDktd2Via2l0LWJvcmRlci1yYWRpdXMlM0E0cHglM0IlMEElMDklMDklMDktbW96LWJvcmRlci1yYWRpdXMlM0E0cHglM0IlMEElMDklMDklMDktbXMtYm9yZGVyLXJhZGl1cyUzQTRweCUzQiUwQSUwOSUwOSUwOS1vLWJvcmRlci1yYWRpdXMlM0E0cHglM0IlMEElMDklMDklMDlib3JkZXItcmFkaXVzJTNBNHB4JTNCJTBBJTA5JTA5JTA5cGFkZGluZyUzQTEwcHglMjAyMHB4JTNCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjNmZmZmZmYlM0IlMEElMDklMDklN0QlMEElMDklMDkuZmVhdHVyZS1yZWFkbW9yZSU3QiUwQSUwOSUwOSUwOXBhZGRpbmctdG9wJTNBN3B4JTNCJTBBJTA5JTA5JTA5cGFkZGluZy1ib3R0b20lM0E0MHB4JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmZlYXR1cmUtdGl0bGUtd3JhcCU3QiUwQSUwOSUwOSUwOXBhZGRpbmctdG9wJTNBMCUzQiUwQSUwOSUwOSUwOXBhZGRpbmctYm90dG9tJTNBMCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5lbnRyeSUyMGgzJTdCJTBBJTA5JTA5JTA5Zm9udC1zaXplJTNBMTdweCUzQiUwQSUwOSUwOSUwOWZvbnQtd2VpZ2h0JTNBbm9ybWFsJTNCJTBBJTA5JTA5JTA5bWFyZ2luJTNBMTVweCUyMDAlMjA1cHglM0IlMEElMDklMDklMDlwYWRkaW5nJTNBMCUzQiUwQSUwOSUwOSUwOWNvbG9yJTNBJTIzMzMzMzMzJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmVudHJ5JTIwaDMlMjBhJTdCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjMzMzMzMzMlM0IlMEElMDklMDklMDl0ZXh0LWRlY29yYXRpb24lM0Fub25lJTNCJTBBJTA5JTA5JTA5Zm9udC13ZWlnaHQlM0E3MDAlM0IlMEElMDklMDklN0QlMEElMDklMDkuZW50cnklMjAudGFnJTdCJTBBJTA5JTA5JTA5Zm9udC13ZWlnaHQlM0E3MDAlM0IlMEElMDklMDklMDlmb250LXNpemUlM0ExM3B4JTNCJTBBJTA5JTA5JTA5dGV4dC10cmFuc2Zvcm0lM0F1cHBlcmNhc2UlM0IlMEElMDklMDklMDljb2xvciUzQSUyM2ZmOTAwZSUzQiUwQSUwOSUwOSUwOW1hcmdpbi1ib3R0b20lM0E1cHglM0IlMEElMDklMDklMDlkaXNwbGF5JTNBYmxvY2slM0IlMEElMDklMDklN0QlMEElMDklMDkuZW50cnklMjBwJTdCJTBBJTA5JTA5JTA5Zm9udC1zaXplJTNBMTRweCUzQiUwQSUwOSUwOSUwOWNvbG9yJTNBJTIzNjY2NjY2JTNCJTBBJTA5JTA5JTA5bGluZS1oZWlnaHQlM0ExLjUlM0IlMEElMDklMDklMDltYXJnaW4tdG9wJTNBMCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5lbnRyeSUyMGEubW9yZSU3QiUwQSUwOSUwOSUwOWNvbG9yJTNBJTIzZmY5MDBlJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmxvZ28tc29jaWFsJTIwcCUyQy5lbWFpbC1mb290ZXIlMjBwJTdCJTBBJTA5JTA5JTA5cGFkZGluZyUzQTAlM0IlMEElMDklMDklMDltYXJnaW4lM0EwJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmVtYWlsLWZvb3RlciUyMHAlN0IlMEElMDklMDklMDljb2xvciUzQSUyM2ZmZmZmZiUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS50aXRsZSU3QiUwQSUwOSUwOSUwOWZvbnQtc2l6ZSUzQTE0cHglM0IlMEElMDklMDklMDlsZXR0ZXItc3BhY2luZyUzQTNweCUzQiUwQSUwOSUwOSUwOXRleHQtdHJhbnNmb3JtJTNBdXBwZXJjYXNlJTNCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjNmMDZkMjIlM0IlMEElMDklMDklMDl0ZXh0LWFsaWduJTNBY2VudGVyJTNCJTBBJTA5JTA5JTA5Zm9udC13ZWlnaHQlM0Fub3JtYWwlM0IlMEElMDklMDklN0QlMEElMDklMDkuZmVhdHVyZS10aXRsZSU3QiUwQSUwOSUwOSUwOWZvbnQtc2l6ZSUzQTMwcHglM0IlMEElMDklMDklMDltYXJnaW4lM0EwJTIwMCUyMDVweCUzQiUwQSUwOSUwOSUwOXBhZGRpbmclM0EwJTNCJTBBJTA5JTA5JTA5Zm9udC13ZWlnaHQlM0Fub3JtYWwlM0IlMEElMDklMDklN0QlMEElMDklMDkuZmVhdHVyZS10aXRsZSUyMHN0cm9uZyU3QiUwQSUwOSUwOSUwOWZvbnQtd2VpZ2h0JTNBYm9sZCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5mZWF0dXJlLWRlc2MlN0IlMEElMDklMDklMDlmb250LXNpemUlM0ExNnB4JTNCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjM4ZThlOGUlM0IlMEElMDklMDklMDltYXJnaW4lM0EwJTNCJTBBJTA5JTA5JTA5cGFkZGluZyUzQTAlM0IlMEElMDklMDklN0QlMEElMDklMDkuYXV0aG9yLWRhdGUlMjAuYXV0aG9yJTJDLmF1dGhvci1kYXRlJTIwLmRhdGUlN0IlMEElMDklMDklMDlkaXNwbGF5JTNBYmxvY2slM0IlMEElMDklMDklMDlmb250LXNpemUlM0ExOHB4JTNCJTBBJTA5JTA5JTA5bGluZS1oZWlnaHQlM0EyNHB4JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmF1dGhvci1kYXRlJTIwLmRhdGUlN0IlMEElMDklMDklMDljb2xvciUzQSUyM2IwYjBiMCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5zbWFsbC1yc3ZwLWJ1dHRvbiUyMC5yc3ZwJTdCJTBBJTA5JTA5JTA5cGFkZGluZyUzQTRweCUyMDEwcHglM0IlMEElMDklMDklMDliYWNrZ3JvdW5kJTNBJTIzZjA2ZDIyJTNCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjNmZmZmZmYlM0IlMEElMDklMDklMDl0ZXh0LWRlY29yYXRpb24lM0Fub25lJTNCJTBBJTA5JTA5JTA5Zm9udC1zaXplJTNBMTJweCUzQiUwQSUwOSUwOSUwOXRleHQtdHJhbnNmb3JtJTNBdXBwZXJjYXNlJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LnRhbGstZGF0ZSU3QiUwQSUwOSUwOSUwOXBhZGRpbmctdG9wJTNBMTBweCUzQiUwQSUwOSUwOSUwOXBhZGRpbmctYm90dG9tJTNBMTBweCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS50YWxrLWRhdGUlMjAuYXV0aG9yJTJDLnRhbGstZGF0ZSUyMC5kYXRlJTdCJTBBJTA5JTA5JTA5ZGlzcGxheSUzQWJsb2NrJTNCJTBBJTA5JTA5JTA5Zm9udC1zaXplJTNBMTRweCUzQiUwQSUwOSUwOSUwOWxpbmUtaGVpZ2h0JTNBMjBweCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS50YWxrLWRhdGUlMjAuZGF0ZSU3QiUwQSUwOSUwOSUwOWNvbG9yJTNBJTIzYjBiMGIwJTNCJTBBJTA5JTA5JTA5Zm9udC1zaXplJTNBMTJweCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5zbWFsbC1yc3ZwLWJ1dHRvbiU3QiUwQSUwOSUwOSUwOXBhZGRpbmclM0ExNXB4JTIwMCUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5iaWctcnN2cC1idXR0b24lMjAucnN2cCU3QiUwQSUwOSUwOSUwOWJhY2tncm91bmQlM0ElMjNmMDZkMjIlM0IlMEElMDklMDklMDlwYWRkaW5nJTNBMTVweCUyMDMwcHglM0IlMEElMDklMDklMDljb2xvciUzQSUyM2ZmZmZmZiUzQiUwQSUwOSUwOSUwOXRleHQtZGVjb3JhdGlvbiUzQW5vbmUlM0IlMEElMDklMDklMDl0ZXh0LXRyYW5zZm9ybSUzQXVwcGVyY2FzZSUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS5wYW5lbGlzdCUyMC5wYW5lbCU3QiUwQSUwOSUwOSUwOXdpZHRoJTNBMjAlMjUlM0IlMEElMDklMDklN0QlMEElMDklMDkucGFuZWxpc3QlMjAucGFuZWwubGFzdCU3QiUwQSUwOSUwOSUwOW1hcmdpbi1yaWdodCUzQTAlM0IlMEElMDklMDklN0QlMEElMDklMDkucGFuZWxpc3QlMjAucGFuZWwlMjBpbWclN0IlMEElMDklMDklMDltYXgtd2lkdGglM0ExMDAlMjUlM0IlMEElMDklMDklMDlkaXNwbGF5JTNBYmxvY2slM0IlMEElMDklMDklN0QlMEElMDklMDkucGFuZWxpc3QlMjAucGFuZWwlMjBwJTdCJTBBJTA5JTA5JTA5bWFyZ2luLWJvdHRvbSUzQTAlM0IlMEElMDklMDklN0QlMEElMDklMDlkaXYubTE2MDU0ZWM4MTViYWMzMDclMjBhJTIwaW1nJTdCJTBBJTA5JTA5JTA5bWFyZ2luLXRvcCUzQS0zcHglMjAlMjFpbXBvcnRhbnQlM0IlMEElMDklMDklN0QlMEElMDklNDBtZWRpYSUyMG9ubHklMjBzY3JlZW4lMjBhbmQlMjAlMjhtYXgtd2lkdGglM0ElMjA0ODBweCUyOSU3QiUwQSUwOSUwOS5wYW5lbGlzdCUyMC5wYW5lbCUyMHAlN0IlMEElMDklMDklMDlmb250LXNpemUlM0ExMHB4JTNCJTBBJTA5JTA5JTdEJTBBJTBBJTdEJTA5JTQwbWVkaWElMjBvbmx5JTIwc2NyZWVuJTIwYW5kJTIwJTI4bWF4LXdpZHRoJTNBJTIwNDgwcHglMjklN0IlMEElMDklMDkubS1sZWZ0JTdCJTBBJTA5JTA5JTA5dGV4dC1hbGlnbiUzQWxlZnQlM0IlMEElMDklMDklMDlwYWRkaW5nLXRvcCUzQTAlM0IlMEElMDklMDklN0QlMEElMEElN0QlMDklNDBtZWRpYSUyMG9ubHklMjBzY3JlZW4lMjBhbmQlMjAlMjhtYXgtd2lkdGglM0ElMjA0ODBweCUyOSU3QiUwQSUwOSUwOXRkLnplcm8lN0IlMEElMDklMDklMDlwYWRkaW5nLWxlZnQlM0E1cHglMjAlMjFpbXBvcnRhbnQlM0IlMEElMDklMDklN0QlMEElMEElN0QlMDklNDBtZWRpYSUyMG9ubHklMjBzY3JlZW4lMjBhbmQlMjAlMjhtYXgtd2lkdGglM0ElMjA0ODBweCUyOSU3QiUwQSUwOSUwOS5lbnRyeSUyMHRkJTdCJTBBJTA5JTA5JTA5d2lkdGglM0ExMDAlMjUlMjAlMjFpbXBvcnRhbnQlM0IlMEElMDklMDklMDlkaXNwbGF5JTNBYmxvY2slMjAlMjFpbXBvcnRhbnQlM0IlMEElMDklMDklN0QlMEElMEElN0QlMDklNDBtZWRpYSUyMG9ubHklMjBzY3JlZW4lMjBhbmQlMjAlMjhtYXgtd2lkdGglM0ElMjA0ODBweCUyOSU3QiUwQSUwOSUwOS5lbnRyeS10YWJsZSU3QiUwQSUwOSUwOSUwOXBhZGRpbmclM0EwJTIwMTVweCUyMCUyMWltcG9ydGFudCUzQiUwQSUwOSUwOSU3RCUwQSUwQSU3RCUwOSU0MG1lZGlhJTIwb25seSUyMHNjcmVlbiUyMGFuZCUyMCUyOG1heC13aWR0aCUzQSUyMDQ4MHB4JTI5JTdCJTBBJTA5JTA5JTIzZW1haWxIZWFkZXIlN0IlMEElMDklMDklMDlwYWRkaW5nJTNBMCUyMCUyMWltcG9ydGFudCUzQiUwQSUwOSUwOSUwOWJvcmRlci1zcGFjaW5nJTNBMCUzQiUwQSUwOSUwOSUwOWJvcmRlci1jb2xsYXBzZSUzQXNlcGFyYXRlJTNCJTBBJTA5JTA5JTdEJTBBJTBBJTdEJTA5JTQwbWVkaWElMjBvbmx5JTIwc2NyZWVuJTIwYW5kJTIwJTI4bWF4LXdpZHRoJTNBJTIwNDgwcHglMjklN0IlMEElMDklMDkuYm9keVRhYmxlJTdCJTBBJTA5JTA5JTA5Ym9yZGVyLXNwYWNpbmclM0EwJTIwJTIxaW1wb3J0YW50JTNCJTBBJTA5JTA5JTA5Ym9yZGVyLWNvbGxhcHNlJTNBY29sbGFwc2UlMjAlMjFpbXBvcnRhbnQlM0IlMEElMDklMDklN0QlMEElMEElN0QlMDklMDkudGFsayUyMGltZyU3QiUwQSUwOSUwOSUwOW1heC13aWR0aCUzQTEwMCUyNSUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOS50YWxrJTIwaDQlN0IlMEElMDklMDklMDljb2xvciUzQSUyM2YwNmQyMiUzQiUwQSUwOSUwOSUwOXRleHQtdHJhbnNmb3JtJTNBdXBwZXJjYXNlJTNCJTBBJTA5JTA5JTA5Zm9udC1zaXplJTNBMTNweCUzQiUwQSUwOSUwOSUwOW1hcmdpbiUzQTAlM0IlMEElMDklMDklMDlwYWRkaW5nJTNBMCUzQiUwQSUwOSUwOSUwOXRleHQtYWxpZ24lM0FsZWZ0JTNCJTBBJTA5JTA5JTA5bWFyZ2luLWJvdHRvbSUzQTEwcHglM0IlMEElMDklMDklN0QlMEElMDklMDkudGFsa3Mtc21hbGwlMjAucGFuZWxpc3Qtc21hbGwlMjAucGFuZWwlN0IlMEElMDklMDklMDl3aWR0aCUzQTIwJTI1JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LnRhbGtzLXNtYWxsJTIwLnBhbmVsaXN0LXNtYWxsJTIwLnBhbmVsJTIwcCU3QiUwQSUwOSUwOSUwOWZvbnQtc2l6ZSUzQTEwcHglM0IlMEElMDklMDklN0QlMEElMDklMDkudGFsa3Mtc21hbGwlMjAucGFuZWxpc3Qtc21hbGwlMjAucGFuZWwlMjBwJTNBbGFzdC1jaGlsZCU3QiUwQSUwOSUwOSUwOW1hcmdpbi1ib3R0b20lM0EwJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LnRhbGtzLXNtYWxsJTIwLnBhbmVsaXN0LXNtYWxsJTIwLnBhbmVsJTIwaW1nJTdCJTBBJTA5JTA5JTA5bWF4LXdpZHRoJTNBMTAwJTI1JTNCJTBBJTA5JTA5JTA5ZGlzcGxheSUzQWJsb2NrJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LnRhbGtzLXNtYWxsJTIwLnBhbmVsaXN0LXNtYWxsJTIwLnBhbmVsJTIwYSU3QiUwQSUwOSUwOSUwOXBhZGRpbmclM0EzcHglM0IlMEElMDklMDklMDlkaXNwbGF5JTNBYmxvY2slM0IlMEElMDklMDklN0QlMEElMDklMDkuY2FsbC10by1hY3Rpb24lN0IlMEElMDklMDklMDlwYWRkaW5nJTNBMjBweCUzQiUwQSUwOSUwOSUwOXRleHQtYWxpZ24lM0FjZW50ZXIlM0IlMEElMDklMDklMDliYWNrZ3JvdW5kJTNBJTIzZWJlYmViJTNCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjNhNmE2YTYlM0IlMEElMDklMDklMDlmb250LXNpemUlM0ExM3B4JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmNhbGwtdG8tYWN0aW9uJTIwYSU3QiUwQSUwOSUwOSUwOWNvbG9yJTNBJTIzZjA2ZDIyJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LnRhbGstdGl0bGUlN0IlMEElMDklMDklMDltYXJnaW4tdG9wJTNBMTBweCUzQiUwQSUwOSUwOSUwOXRleHQtYWxpZ24lM0FsZWZ0JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LnRhbGstdGl0bGUlMjBhJTdCJTBBJTA5JTA5JTA5Y29sb3IlM0ElMjNmMDZkMjIlM0IlMEElMDklMDklMDlmb250LXN0eWxlJTNBaXRhbGljJTNCJTBBJTA5JTA5JTA5Zm9udC1zaXplJTNBMThweCUzQiUwQSUwOSUwOSUwOWZvbnQtd2VpZ2h0JTNBbm9ybWFsJTNCJTBBJTA5JTA5JTA5dGV4dC1kZWNvcmF0aW9uJTNBdW5kZXJsaW5lJTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LnRleHRDZW50ZXIlN0IlMEElMDklMDklMDl0ZXh0LWFsaWduJTNBY2VudGVyJTIwJTIxaW1wb3J0YW50JTNCJTBBJTA5JTA5JTdEJTBBJTA5JTA5LmVtYWlsLWZvb3RlciUyMGElN0IlMEElMDklMDklMDljb2xvciUzQSUyM2ZmZmZmZiUzQiUwQSUwOSUwOSU3RCUwQSUwOSUwOSUyM3RvcC1nYXAlN0IlMEElMDklMDklMDltYXJnaW4tdG9wJTNBLTRweCUyMCUyMWltcG9ydGFudCUzQiUwQSUwOSUwOSU3RCUwQSUzQyUyRnN0eWxlJTNFJTNDJTJGaGVhZCUzRSUwQSUyMCUyMCUzQ2JvZHklM0UlMEElMjAlMjAlMjAlMjAlM0N0YWJsZSUyMGJvcmRlciUzRCUyMjAlMjIlMjBjZWxscGFkZGluZyUzRCUyMjAlMjIlMjBjZWxsc3BhY2luZyUzRCUyMjAlMjIlMjB3aWR0aCUzRCUyMjEwMCUyNSUyMiUyMGNsYXNzJTNEJTIyYm9keVRhYmxlJTIyJTIwc3R5bGUlM0QlMjJoZWlnaHQlM0ExMDAlMjUlM0Jib3JkZXItYm90dG9tJTNBMHB4JTNCJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTIwdmFsaWduJTNEJTIydG9wJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjI2ODAlMjIlMjBjbGFzcyUzRCUyMmVtYWlsQ29udGFpbmVyJTIwbWFpbC1jb250YWluZXIlMjIlMjBzdHlsZSUzRCUyMmJhY2tncm91bmQlM0ElMjNGRkYlM0Jib3JkZXIlM0ExcHglMjBzb2xpZCUyMCUyM2YwZjBmMCUzQmJvcmRlci1ib3R0b20lM0EwcHglM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUyMGJnY29sb3IlM0QlMjIlMjNGRkZGRkYlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTIwdmFsaWduJTNEJTIydG9wJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIyMCUyMiUyMGNlbGxzcGFjaW5nJTNEJTIyMCUyMiUyMHdpZHRoJTNEJTIyMTAwJTI1JTIyJTIwaWQlM0QlMjJlbWFpbEhlYWRlciUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjBhbGlnbiUzRCUyMmxlZnQlMjIlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBjbGFzcyUzRCUyMmNvbDItMSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cCUzQSUyRiUyRnRyaWFseC5jb20lMkYlMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlM0UlM0NpbWclMjBzcmMlM0QlMjJodHRwcyUzQSUyRiUyRmdhbGxlcnkubWFpbGNoaW1wLmNvbSUyRjE5ODhkMTE2YzQ0NDc1NjRjNThlMDNhNWUlMkZpbWFnZXMlMkY0YmJmZDI2OS0xYTYzLTRkYzYtOTcxYi0yODdiYTBmZTdjZmIucG5nJTIyJTIwYWx0JTNEJTIyJTIyJTIwYm9yZGVyJTNEJTIyMCUyMiUyMHN0eWxlJTNEJTIybWFyZ2luJTNBJTIwMCUzQiUyMHBhZGRpbmclM0ElMjAwJTNCJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm5pbWcwMSUyMiUzRSUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwd2lkdGglM0QlMjI1MCUyNSUyMiUyMGFsaWduJTNEJTIycmlnaHQlMjIlMjBjbGFzcyUzRCUyMmNvbDItMSUyMG0tbGVmdCUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGlkJTNEJTIyZGF0ZS10b3AlMjIlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQwMSUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjM5ODk4OTclM0IlMjIlM0VzZXB0ZW1iZXIlMjAyMCUyQyUyMDIwMTglM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGFibGUlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTIwdmFsaWduJTNEJTIydG9wJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDaW1nJTIwc3JjJTNEJTIyaHR0cHMlM0ElMkYlMkZnYWxsZXJ5Lm1haWxjaGltcC5jb20lMkYxOTg4ZDExNmM0NDQ3NTY0YzU4ZTAzYTVlJTJGaW1hZ2VzJTJGNTMwODU5M2MtNmM1OS00MDE5LThhNDAtNTUzMGM4MjdiMmM2LmpwZyUyMiUyMGFsdCUzRCUyMiUyMiUyMGJvcmRlciUzRCUyMjAlMjIlMjBzdHlsZSUzRCUyMm1hcmdpbiUzQSUyMDAlM0IlMjBwYWRkaW5nJTNBJTIwMCUzQiUyMiUyMG1jJTNBZWRpdCUzRCUyMmJhbm5lcjElMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjI2ODAlMjIlMjBjbGFzcyUzRCUyMmVtYWlsQ29udGFpbmVyLXdoaXRlJTIwbWFpbC1jb250YWluZXIlMjIlMjBzdHlsZSUzRCUyMmJvcmRlci1ib3R0b20lM0EwcHglM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJjZW50ZXIlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0YWJsZSUyMGJvcmRlciUzRCUyMjAlMjIlMjBjZWxscGFkZGluZyUzRCUyMjAlMjIlMjBjZWxsc3BhY2luZyUzRCUyMjAlMjIlMjB3aWR0aCUzRCUyMjEwMCUyNSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmxlZnQlMjIlMjBzdHlsZSUzRCUyMnBhZGRpbmclM0EwcHglMjAxNXB4JTIwMCUyMDQ1cHglM0JwYWRkaW5nLWJvdHRvbSUzQTUwcHglM0IlMjIlMjBjbGFzcyUzRCUyMmVudHJ5LXRhYmxlJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIxMCUyMiUyMGNlbGxzcGFjaW5nJTNEJTIyMCUyMiUyMHdpZHRoJTNEJTIyMTAwJTI1JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTIwY29sc3BhbiUzRCUyMjIlMjIlMjBzdHlsZSUzRCUyMnBhZGRpbmctYm90dG9tJTNBNDBweCUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2gyJTIwY2xhc3MlM0QlMjJoZWFkaW5nLTIlMjIlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQwNSUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjMzMzMzMzMlMjAlMjFpbXBvcnRhbnQlM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NzdHJvbmclM0UlMjBGcm9tJTIwdGhlJTIwVHJpYWxYJTIwRGVzayUzQyUyRnN0cm9uZyUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmgyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDaW1nJTIwc3JjJTNEJTIyaHR0cHMlM0ElMkYlMkZnYWxsZXJ5Lm1haWxjaGltcC5jb20lMkY1MDBkMGI1MjM2MjMyNTJlNGRlYjM1MTgzJTJGaW1hZ2VzJTJGOWQ1YjU5ZDItZDc1Yy00YzJmLTg3NjMtNDg0NzdiY2QxZmQ1LnBuZyUyMiUyMGFsdCUzRCUyMiUyMiUyMHN0eWxlJTNEJTIyZGlzcGxheSUzQSUyMGJsb2NrJTNCJTIwbWF4LXdpZHRoJTNBJTIwMTAwJTI1JTNCJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTIwY2xhc3MlM0QlMjJlbnRyeSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwd2lkdGglM0QlMjIzNSUyNSUyMiUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGNsYXNzJTNEJTIyemVybyUyMiUyMHN0eWxlJTNEJTIycGFkZGluZyUzQTAlM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NpbWclMjBzcmMlM0QlMjJodHRwcyUzQSUyRiUyRmdhbGxlcnkubWFpbGNoaW1wLmNvbSUyRjE5ODhkMTE2YzQ0NDc1NjRjNThlMDNhNWUlMkZpbWFnZXMlMkYxOGNjMjQwOS1kZWNiLTQ4NDQtOTU3My00MDA1MGRlMGU5OGEuanBnJTIyJTIwYWx0JTNEJTIyJTIyJTIwYm9yZGVyJTNEJTIyMCUyMiUyMHN0eWxlJTNEJTIybWFyZ2luJTNBJTIwMCUzQiUyMHBhZGRpbmclM0ElMjAwJTNCJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm5pbWcwNCUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjB3aWR0aCUzRCUyMjY1JTI1JTIyJTIwdmFsaWduJTNEJTIydG9wJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUyMGNsYXNzJTNEJTIydGFnJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm50ZXh0MDYlMjIlM0VDdXJlVGFsa3MlM0MlMkZzcGFuJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDaDMlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQwNyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZ3d3cuY3VyZXRhbGtzLmNvbSUyRmV2ZW50JTJGcnN2cCUyRkFsemhlaW1lci1zLW9yLURlbWVudGlhLVdoYXQtcy10aGUtRGlmZmVyZW5jZS0lMkYzMDklMkYlM0Z1cGNvbWluZyUzRHllcyUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjMzMzMzMzMlMjAlMjFpbXBvcnRhbnQlM0IlMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlM0VBbHpoZWltZXIlMjdzJTIwb3IlMjBEZW1lbnRpYS4lM0NiciUzRSUyMFdoYXQlMjdzJTIwdGhlJTIwRGlmZmVyZW5jZSUzRiUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZoMyUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3AlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQwOCUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjM2NjY2NjYlMjAlMjFpbXBvcnRhbnQlM0JwYWRkaW5nLXJpZ2h0JTNBMTVweCUzQiUyMiUzRSUzQ3N0cm9uZyUzRURyJTIwTXVycmF5JTIwR3Jvc3NtYW4lM0MlMkZzdHJvbmclM0UlMjAlMjZhbXAlM0IlM0NzdHJvbmclM0UlMjBEciUyMERhdmlkJTIwV29sayUzQyUyRnN0cm9uZyUzRSUyMG9mJTIwUGVubiUyME1lZGljaW5lJTIwY2xlYXIlMjB0aGUlMjBhaXIlMjBhYm91dCUyMEFsemhlaW1lciVFMiU4MCU5OXMlMjAlMjZhbXAlM0IlMjBGcm9udG90ZW1wb3JhbCUyMGRlbWVudGlhJTIwJTIzRlREJTIwLSUyMHR3byUyMHNpbWlsYXIlMjBidXQlMjB2ZXJ5JTIwZGlmZmVyZW50JTIwZGlzZWFzZXMuJTNDJTJGcCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3AlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQwOSUyMiUzRSUzQ2ElMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZ3d3cuY3VyZXRhbGtzLmNvbSUyRmV2ZW50JTJGcnN2cCUyRkFsemhlaW1lci1zLW9yLURlbWVudGlhLVdoYXQtcy10aGUtRGlmZmVyZW5jZS0lMkYzMDklMkYlM0Z1cGNvbWluZyUzRHllcyUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUyMGNsYXNzJTNEJTIybW9yZSUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjNmZjkwMGUlMjAlMjFpbXBvcnRhbnQlM0IlMjIlM0VSZWFkJTIwbW9yZSUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZwJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTIwY2xhc3MlM0QlMjJlbnRyeSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwd2lkdGglM0QlMjIzNSUyNSUyMiUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGNsYXNzJTNEJTIyemVybyUyMiUyMHN0eWxlJTNEJTIycGFkZGluZyUzQTAlM0JwYWRkaW5nLXRvcCUzQTQwcHglM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NpbWclMjBzcmMlM0QlMjJodHRwcyUzQSUyRiUyRmdhbGxlcnkubWFpbGNoaW1wLmNvbSUyRjE5ODhkMTE2YzQ0NDc1NjRjNThlMDNhNWUlMkZpbWFnZXMlMkYzMGUwZDdkYi1lZDgyLTRjNmYtYWU5Ny0wMWZhMjE0M2YzYjIuanBnJTIyJTIwYWx0JTNEJTIyJTIyJTIwYm9yZGVyJTNEJTIyMCUyMiUyMHN0eWxlJTNEJTIybWFyZ2luJTNBJTIwMCUzQiUyMHBhZGRpbmclM0ElMjAwJTNCJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm5pbWcwNSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjB3aWR0aCUzRCUyMjY1JTI1JTIyJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwc3R5bGUlM0QlMjJwYWRkaW5nJTNBMCUzQnBhZGRpbmctdG9wJTNBNDBweCUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NwYW4lMjBjbGFzcyUzRCUyMnRhZyUyMiUyMG1jJTNBZWRpdCUzRCUyMmljb25udGV4dDEwJTIyJTNFQ09ORkVSRU5DRSUzQyUyRnNwYW4lM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NoMyUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cCUzQSUyRiUyRnRyaWFseC5jb20lMkZwcmVzZW50aW5nLWFwcGJha2VyeS0yLTAtYXQtbW9iaWxlLWluLWNsaW5pY2FsLXRyaWFscy0yMDE4LWNvbmZlcmVuY2UlMkYlMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQxMSUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjMzMzMzMzMlMjAlMjFpbXBvcnRhbnQlM0IlMjIlM0VQcmVzZW50aW5nJTIwQXBwYmFrZXJ5JTIwMi4wJTIwYXQlMjBNb2JpbGUlMjAlM0NiciUzRSUyMGluJTIwQ2xpbmljYWwlMjBUcmlhbHMlMjAyMDE4JTIwQ29uZmVyZW5jZSUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZoMyUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3AlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQxMiUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjM2NjY2NjYlMjAlMjFpbXBvcnRhbnQlM0JwYWRkaW5nLXJpZ2h0JTNBMTVweCUzQiUyMiUzRUxlYXJuJTIwaG93JTIwJTIwQXBwYmFrZXJ5JTIwMi4wJTIwJTIwY2FuJTIwc3VwcG9ydCUyMHlvdXIlMjByZXNlYXJjaCUyMHN0dWR5JTIwZGF0YSUyMGNvbGxlY3Rpb24lMjBieSUyMGhlbHBpbmclMjB5b3UlMjBidWlsZCUyMHlvdXIlMjBvd24lMjBzdHVkeSUyMEFwcC4lM0MlMkZwJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDcCUyMG1jJTNBZWRpdCUzRCUyMmljb25udGV4dDEzJTIyJTNFJTNDYSUyMGhyZWYlM0QlMjJodHRwJTNBJTJGJTJGdHJpYWx4LmNvbSUyRnByZXNlbnRpbmctYXBwYmFrZXJ5LTItMC1hdC1tb2JpbGUtaW4tY2xpbmljYWwtdHJpYWxzLTIwMTgtY29uZmVyZW5jZSUyRiUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUyMGNsYXNzJTNEJTIybW9yZSUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjNmZjkwMGUlMjAlMjFpbXBvcnRhbnQlM0IlMjIlM0VSZWFkJTIwbW9yZSUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZwJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRhYmxlJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRhYmxlJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRhYmxlJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmxlZnQlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0YWJsZSUyMGJvcmRlciUzRCUyMjAlMjIlMjBjZWxscGFkZGluZyUzRCUyMjIwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjIxMDAlMjUlMjIlMjBzdHlsZSUzRCUyMmJhY2tncm91bmQlM0ElMjNmMGYwZjAlM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjIxMDAlMjUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJjZW50ZXIlMjIlMjBjb2xzcGFuJTNEJTIyMiUyMiUyMGNsYXNzJTNEJTIyZmVhdHVyZS10aXRsZS13cmFwJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDaDElMjBjbGFzcyUzRCUyMmZlYXR1cmUtdGl0bGUlMjIlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQwMiUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjMzMzMzMzMlMjAlMjFpbXBvcnRhbnQlM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NzdHJvbmclM0VBcHBiYWtlcnklMjBVcGRhdGVzJTNDJTJGc3Ryb25nJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGaDElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NwJTIwY2xhc3MlM0QlMjJmZWF0dXJlLWRlc2MlMjIlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQwMyUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjM2NjY2NjYlMjAlMjFpbXBvcnRhbnQlM0J3aWR0aCUzQTcyJTI1JTNCJTIyJTNFQXBwYmFrZXJ5JTIwY2FuJTIwaGVscCUyMHlvdSUyMGxhdW5jaCUyMHlvdXIlMjByZXNlYXJjaCUyMHN0dWR5JTIwYXBwcyUyMGluJTIwbm8lMjB0aW1lJTIwYW5kJTIwYXQlMjBsb3clMjBjb3N0JTNDJTJGcCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0YWJsZSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0YWJsZSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUyMHN0eWxlJTNEJTIyYmFja2dyb3VuZCUzQSUyM2YwZjBmMCUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJjZW50ZXIlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwaHJlZiUzRCUyMmh0dHAlM0ElMkYlMkZ0cmlhbHguY29tJTJGYXBwYmFrZXJ5JTJGJTIyJTIwdGFyZ2V0JTNEJTIyX2JsYW5rJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm5pbWcwMyUyMiUzRSUzQ2ltZyUyMGJvcmRlciUzRCUyMjAlMjIlMjBoZWlnaHQlM0QlMjIxNDAlMjIlMjBzcmMlM0QlMjJodHRwcyUzQSUyRiUyRmdhbGxlcnkubWFpbGNoaW1wLmNvbSUyRjE5ODhkMTE2YzQ0NDc1NjRjNThlMDNhNWUlMkZpbWFnZXMlMkY1NWI0ODliMS1lNDEyLTRiMjUtYjRmNS1iNzU3ZWZlNzcxZWIuanBnJTIyJTIwc3R5bGUlM0QlMjJib3JkZXIlM0ElMjAwcHglMjAlMjAlM0IlMjBwYWRkaW5nJTNBJTIwMHB4JTNCJTIwd2lkdGglM0ElMjA1ODRweCUzQiUyMGhlaWdodCUzQSUyMDE0MHB4JTNCJTIwbWFyZ2luJTNBJTIwMHB4JTNCJTIyJTIwd2lkdGglM0QlMjI1ODQlMjIlMjBhbHQlM0QlMjIwODc3NTJiYi1hM2IxLTQ0NmQtYjQzMi0wNDcwMWE3ZTMyOWYuanBnJTIyJTNFJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUyMHN0eWxlJTNEJTIyYmFja2dyb3VuZCUzQSUyM2YwZjBmMCUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJjZW50ZXIlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwaHJlZiUzRCUyMmh0dHAlM0ElMkYlMkZ0cmlhbHguY29tJTJGYXBwYmFrZXJ5JTJGJTIyJTIwdGFyZ2V0JTNEJTIyX2JsYW5rJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm5pbWcwMzExJTIyJTNFJTNDaW1nJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGhlaWdodCUzRCUyMjE0MCUyMiUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGZ2FsbGVyeS5tYWlsY2hpbXAuY29tJTJGMTk4OGQxMTZjNDQ0NzU2NGM1OGUwM2E1ZSUyRmltYWdlcyUyRjdhOTMwZWZhLWE4ZmItNGE3Mi05ZDAyLWEyZGIyZmIwNDk2Ni5qcGclMjIlMjBzdHlsZSUzRCUyMmJvcmRlciUzQSUyMDBweCUyMCUyMCUzQiUyMHBhZGRpbmclM0ElMjAwcHglM0IlMjB3aWR0aCUzQSUyMDU4NHB4JTNCJTIwaGVpZ2h0JTNBJTIwMTQwcHglM0IlMjBtYXJnaW4lM0ElMjAwcHglM0IlMjIlMjB3aWR0aCUzRCUyMjU4NCUyMiUyMGFsdCUzRCUyMjk1ZGFlYzc0LTNkMzAtNDZiNS04NzNhLTE5ZTVlNzJjYWQxMi5qcGclMjIlM0UlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTIwc3R5bGUlM0QlMjJiYWNrZ3JvdW5kJTNBJTIzZjBmMGYwJTNCJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmNlbnRlciUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cCUzQSUyRiUyRnRyaWFseC5jb20lMkZ3ZWJpbmFycyUyRnJlc2VhcmNoLWRhdGEtY29sbGVjdGlvbi13aXRoLW1vYmlsZS1zdHVkeS1hcHBzJTJGJTIyJTIwdGFyZ2V0JTNEJTIyX2JsYW5rJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm5pbWcwMzEyJTIyJTNFJTNDaW1nJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGhlaWdodCUzRCUyMjE0MCUyMiUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGZ2FsbGVyeS5tYWlsY2hpbXAuY29tJTJGMTk4OGQxMTZjNDQ0NzU2NGM1OGUwM2E1ZSUyRmltYWdlcyUyRjc2Mzg4NzBlLTMzMDAtNGIzOS04ZjVhLTM0M2E1MjJlZDBmNi5qcGclMjIlMjBzdHlsZSUzRCUyMmJvcmRlciUzQSUyMDBweCUyMGluaXRpYWwlMjAlM0IlMjBwYWRkaW5nJTNBJTIwMHB4JTNCJTIwd2lkdGglM0ElMjA1ODRweCUzQiUyMGhlaWdodCUzQSUyMDE0MHB4JTNCJTIwbWFyZ2luJTNBJTIwMHB4JTNCJTIyJTIwd2lkdGglM0QlMjI1ODQlMjIlMjBhbHQlM0QlMjJiNTIwNzMxZS0zODYzLTRmNDctYWNhZC01YWFiNTBhOTY0NDUuanBnJTIyJTNFJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUyMHN0eWxlJTNEJTIyYmFja2dyb3VuZCUzQSUyM2YwZjBmMCUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJjZW50ZXIlMjIlMjBjbGFzcyUzRCUyMmZlYXR1cmUtcmVhZG1vcmUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGFibGUlM0UlMEElMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlM0N0ZCUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjI2ODAlMjIlMjBjbGFzcyUzRCUyMmVtYWlsQ29udGFpbmVyLWNvbG9yLTIlMjBtYWlsLWNvbnRhaW5lciUyMiUyMHN0eWxlJTNEJTIyYmFja2dyb3VuZCUzQSUyM0ZGRiUzQmJvcmRlciUzQTFweCUyMHNvbGlkJTIwJTIzZjBmMGYwJTNCYm9yZGVyLWJvdHRvbSUzQTBweCUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmNlbnRlciUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RhYmxlJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGNlbGxwYWRkaW5nJTNEJTIyMCUyMiUyMGNlbGxzcGFjaW5nJTNEJTIyMCUyMiUyMHdpZHRoJTNEJTIyMTAwJTI1JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGFsaWduJTNEJTIybGVmdCUyMiUyMHN0eWxlJTNEJTIycGFkZGluZyUzQTBweCUyMDM1cHglMjAzMHB4JTIwMzBweCUzQiUyMiUyMGNsYXNzJTNEJTIyZW50cnktdGFibGUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0YWJsZSUyMGJvcmRlciUzRCUyMjAlMjIlMjBjZWxscGFkZGluZyUzRCUyMjEwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjIxMDAlMjUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwY29sc3BhbiUzRCUyMjIlMjIlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmNlbnRlciUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2gyJTIwY2xhc3MlM0QlMjJoZWFkaW5nLTIlMjIlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQ3MCUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjMzMzMzMzMlMjAlMjFpbXBvcnRhbnQlM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NzdHJvbmclM0VpQ29ubmVjdCUyME5ld3MlM0MlMkZzdHJvbmclM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZoMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ltZyUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGZ2FsbGVyeS5tYWlsY2hpbXAuY29tJTJGNTAwZDBiNTIzNjIzMjUyZTRkZWIzNTE4MyUyRmltYWdlcyUyRjlkNWI1OWQyLWQ3NWMtNGMyZi04NzYzLTQ4NDc3YmNkMWZkNS5wbmclMjIlMjBhbHQlM0QlMjIlMjIlMjBzdHlsZSUzRCUyMmRpc3BsYXklM0ElMjBibG9jayUzQiUyMG1heC13aWR0aCUzQSUyMDEwMCUyNSUzQiUyMG1hcmdpbi1ib3R0b20lM0ElMjAxMHB4JTNCJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDcCUyMGNsYXNzJTNEJTIyZmVhdHVyZS1kZXNjJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm50ZXh0MDQxJTIyJTIwc3R5bGUlM0QlMjJjb2xvciUzQSUyMzY2NjY2NiUyMCUyMWltcG9ydGFudCUzQnBhZGRpbmctdG9wJTNBMTBweCUzQm1hcmdpbi10b3AlM0ExNnB4JTNCbWF4LXdpZHRoJTNBODUlMjUlM0IlMjIlM0VTdGF5JTIwdHVuZWQlMjElM0NiciUzRSUyMGlDb25uZWN0JTIwVmVyc2lvbiUyMDQuMCUyMHdpdGglMjBuZXclMjBmZWF0dXJlcyUyMGNvbWluZyUyMHNvb24lMjElMjAlM0MlMkZwJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjBhbGlnbiUzRCUyMmNlbnRlciUyMiUyMHZhbGlnbiUzRCUyMnRvcCUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cCUzQSUyRiUyRnRyaWFseC5jb20lMkZpY29ubmVjdCUyRiUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUzRSUyMCUzQyUyRmElM0UlM0NhJTIwaHJlZiUzRCUyMmh0dHAlM0ElMkYlMkZ0cmlhbHguY29tJTJGaWNvbm5lY3QlMkYlMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlMjBtYyUzQWVkaXQlM0QlMjJpY29ubmltZzcxJTIyJTNFJTNDaW1nJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGhlaWdodCUzRCUyMjI5NyUyMiUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGZ2FsbGVyeS5tYWlsY2hpbXAuY29tJTJGMTk4OGQxMTZjNDQ0NzU2NGM1OGUwM2E1ZSUyRmltYWdlcyUyRmMwMzZjOTE3LTdlNWItNDdjMi1iNzU1LWYwNzI3MzAwOGZhNy5wbmclMjIlMjBzdHlsZSUzRCUyMmJvcmRlciUzQSUyMDBweCUzQiUyMCUyMCUyMCUyMCUyMCUyMHBhZGRpbmclM0ElMjAwcHglM0IlMjB3aWR0aCUzQSUyMDYwM3B4JTNCJTIwaGVpZ2h0JTNBJTIwMjk3cHglM0IlMjBtYXJnaW4lM0ElMjAwcHglM0IlMjIlMjB3aWR0aCUzRCUyMjYwMyUyMiUyMGFsdCUzRCUyMjFlMzlmZmE4LTc1NzctNGE2MS1hNDk5LTk5MmY3YzFlNzYyNS5wbmclMjIlM0UlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRhYmxlJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRhYmxlJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRhYmxlJTNFJTBBJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTNDdGQlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmNlbnRlciUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RhYmxlJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGNlbGxwYWRkaW5nJTNEJTIyMCUyMiUyMGNlbGxzcGFjaW5nJTNEJTIyMCUyMiUyMHdpZHRoJTNEJTIyNjgwJTIyJTIwY2xhc3MlM0QlMjJlbWFpbENvbnRhaW5lci13aGl0ZSUyMG1haWwtY29udGFpbmVyJTIyJTIwc3R5bGUlM0QlMjJiYWNrZ3JvdW5kJTNBJTIzZjBmMGYwJTNCJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjIxMDAlMjUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJsZWZ0JTIyJTIwc3R5bGUlM0QlMjJwYWRkaW5nJTNBMHB4JTIwMzVweCUyMDAlMjAzNXB4JTNCJTIyJTIwY2xhc3MlM0QlMjJlbnRyeS10YWJsZSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RhYmxlJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGNlbGxwYWRkaW5nJTNEJTIyMTAlMjIlMjBjZWxsc3BhY2luZyUzRCUyMjAlMjIlMjB3aWR0aCUzRCUyMjEwMCUyNSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmNlbnRlciUyMiUyMGNvbHNwYW4lM0QlMjIyJTIyJTIwc3R5bGUlM0QlMjJwYWRkaW5nLWJvdHRvbSUzQTI1cHglM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NoMiUyMGNsYXNzJTNEJTIyaGVhZGluZy0yJTIyJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm50ZXh0MDU1JTIyJTIwc3R5bGUlM0QlMjJjb2xvciUzQSUyMzMzMzMzMyUyMCUyMWltcG9ydGFudCUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3N0cm9uZyUzRVRyZW5kc3BvdHRpbmclMjB3aXRoJTIwVHJpYWxYJTNDJTJGc3Ryb25nJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGaDIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NpbWclMjBzcmMlM0QlMjJodHRwcyUzQSUyRiUyRmdhbGxlcnkubWFpbGNoaW1wLmNvbSUyRjUwMGQwYjUyMzYyMzI1MmU0ZGViMzUxODMlMkZpbWFnZXMlMkY5ZDViNTlkMi1kNzVjLTRjMmYtODc2My00ODQ3N2JjZDFmZDUucG5nJTIyJTIwYWx0JTNEJTIyJTIyJTIwc3R5bGUlM0QlMjJkaXNwbGF5JTNBJTIwYmxvY2slM0IlMjBtYXgtd2lkdGglM0ElMjAxMDAlMjUlM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlMjBjbGFzcyUzRCUyMmVudHJ5MSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwd2lkdGglM0QlMjI1MCUyNSUyMiUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGNsYXNzJTNEJTIyY29sMi0xJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDaW1nJTIwc3JjJTNEJTIyaHR0cHMlM0ElMkYlMkZnYWxsZXJ5Lm1haWxjaGltcC5jb20lMkYxOTg4ZDExNmM0NDQ3NTY0YzU4ZTAzYTVlJTJGaW1hZ2VzJTJGYWRlMDAyMjYtNTVkZC00Y2QzLWE3M2MtYTY0MzliM2ZmMjc2LmpwZyUyMiUyMGFsdCUzRCUyMiUyMiUyMGJvcmRlciUzRCUyMjAlMjIlMjBzdHlsZSUzRCUyMm1hcmdpbiUzQSUyMDAlM0IlMjBwYWRkaW5nJTNBJTIwMCUzQiUyMiUyMG1jJTNBZWRpdCUzRCUyMmljb25uaW1nX3Y0XzA0JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDaDMlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHRfdjRfMDclMjIlMjBzdHlsZSUzRCUyMm1hcmdpbiUzQTE1cHglMjAwJTIwNXB4JTNCZm9udC1zaXplJTNBMTZweCUzQmZvbnQtd2VpZ2h0JTNBNjAwJTNCcGFkZGluZyUzQTAlM0Jjb2xvciUzQSUyMzMzMzMzMyUzQiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cCUzQSUyRiUyRmJpdC5seSUyRjJObEdXaGMlMjIlMjBzdHlsZSUzRCUyMmNvbG9yJTNBJTIzMzMzJTIwJTIxaW1wb3J0YW50JTNCJTIyJTIwdGFyZ2V0JTNEJTIyX2JsYW5rJTIyJTNFUHV0JTIwWW91ciUyMENsaW5pY2FsJTIwVHJpYWxzJTIwRGF0YSUyMHRvJTIwV29yayUyMHdpdGglMjBBcHBsaWVkJTIwTWFjaGluZSUyMExlYXJuaW5nJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmgzJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDcCUyMG1jJTNBZWRpdCUzRCUyMmljb25udGV4dF92NF8wOCUyMiUyMHN0eWxlJTNEJTIyY29sb3IlM0ElMjM2NjY2NjYlMjAlMjFpbXBvcnRhbnQlM0Jmb250LXNpemUlM0ExNXB4JTNCJTIyJTNFQ2xpbmljYWwlMjB0cmlhbHMlMjBkYXRhJTIwaXMlMjBpbmNyZWFzaW5nJTIwaW4lMjBjb21wbGV4aXR5JTIwYXMlMjB3ZWxsJTIwYXMlMjB2b2x1bWUlMjBkYXklMjBieSUyMGRheS4lMjBXaXRoJTIwbGltaXRlZCUyMHRpbWUlMjBhbmQlMjBvdXIlMjBsaW1pdGVkJTIwY2FwYWJpbGl0aWVzJTJDJTIwaXQlMjBpcyUyMG5vdCUyMGh1bWFubHklMjBwb3NzaWJsZSUyMHRvJTIwYW5hbHlzZSUyMHN1Y2glMjBodWdlJTIwZGF0YSUyMHNldHMlMjBjb21wbGV0ZWx5LiUzQyUyRnAlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NwJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm50ZXh0X3Y0XzA5JTIyJTIwc3R5bGUlM0QlMjJtYXJnaW4tdG9wJTNBMTglMjUlM0JtYXJnaW4tYm90dG9tJTNBMTMlMjUlM0IlMjIlM0UlM0NhJTIwY2xhc3MlM0QlMjJtb3JlJTIyJTIwaHJlZiUzRCUyMmh0dHAlM0ElMkYlMkZiaXQubHklMkYyTmxHV2hjJTIyJTIwc3R5bGUlM0QlMjJjb2xvciUzQSUyM2ZmOTAwZSUyMCUyMWltcG9ydGFudCUzQiUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUzRVJlYWQlMjBtb3JlJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnAlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwd2lkdGglM0QlMjI1MCUyNSUyMiUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGNsYXNzJTNEJTIyY29sMi0xJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDaW1nJTIwc3JjJTNEJTIyaHR0cHMlM0ElMkYlMkZnYWxsZXJ5Lm1haWxjaGltcC5jb20lMkYxOTg4ZDExNmM0NDQ3NTY0YzU4ZTAzYTVlJTJGaW1hZ2VzJTJGOTRlYjFhY2QtM2M4Mi00YTFiLTkyNmItNWNiZjBhZmRkMThmLmpwZyUyMiUyMGFsdCUzRCUyMiUyMiUyMGJvcmRlciUzRCUyMjAlMjIlMjBzdHlsZSUzRCUyMm1hcmdpbiUzQSUyMDAlM0IlMjBwYWRkaW5nJTNBJTIwMCUzQiUyMiUyMG1jJTNBZWRpdCUzRCUyMmljb25uaW1nX3Y0XzA0XzIlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NoMyUyMG1jJTNBZWRpdCUzRCUyMmljb25udGV4dF92NF8wN18yJTIyJTIwc3R5bGUlM0QlMjJtYXJnaW4lM0ExNXB4JTIwMCUyMDVweCUzQmZvbnQtc2l6ZSUzQTE2cHglM0Jmb250LXdlaWdodCUzQTYwMCUzQnBhZGRpbmclM0EwJTNCY29sb3IlM0ElMjMzMzMzMzMlM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwaHJlZiUzRCUyMmh0dHAlM0ElMkYlMkZ0cmlhbHguY29tJTJGY3VyZXRhbGslMkYyMDE4JTJGMDklMkYwNyUyRmxldC10aGVyZS1iZS1zaWdodC1yZXZlcnNpbmctYmxpbmRuZXNzLXdpdGgtcmV0aW5hbC1nZW5lLXRoZXJhcHklMkYlMjIlMjBzdHlsZSUzRCUyMmNvbG9yJTNBJTIzMzMzJTIwJTIxaW1wb3J0YW50JTNCJTIyJTIwdGFyZ2V0JTNEJTIyX2JsYW5rJTIyJTNFTGV0JTIwdGhlcmUlMjBiZSUyMFNJR0hUJTIxJTIwJTNDYnIlM0UlMjBSZXZlcnNpbmclMjBCbGluZG5lc3MlMjB3aXRoJTIwUmV0aW5hbCUyMEdlbmUlMjBUaGVyYXB5JTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmgzJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDcCUyMG1jJTNBZWRpdCUzRCUyMmljb25udGV4dF92NF8wOF8yJTIyJTIwc3R5bGUlM0QlMjJjb2xvciUzQSUyMzY2NjY2NiUyMCUyMWltcG9ydGFudCUzQmZvbnQtc2l6ZSUzQTE1cHglM0IlMjIlM0VUaGUlMjBVUyUyMEZEQSUyMGhhcyUyMGFwcHJvdmVkJTIwYSUyMHBhdGhicmVha2luZyUyMGdlbmUlMjB0aGVyYXB5JTIwdHJlYXRtZW50JTIwZm9yJTIwYSUyMGZvcm0lMjBvZiUyMGluaGVyaXRlZCUyMGJsaW5kbmVzcy4lMjBMdXh0dXJuYSUyMGlzJTIwdGhlJTIwZmlyc3QlMjBvZiUyMGFueSUyMHN1Y2glMjB0cmVhdG1lbnQlMjBmb3IlMjBhJTIwZ2VuZXRpYyUyMGNvbmRpdGlvbiUyMGFwcHJvdmVkJTIwaW4lMjB0aGUlMjBVUy4lM0MlMkZwJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDcCUyMG1jJTNBZWRpdCUzRCUyMmljb25udGV4dF92NF8xMCUyMiUyMHN0eWxlJTNEJTIybWFyZ2luLXRvcCUzQTE4JTI1JTNCbWFyZ2luLWJvdHRvbSUzQTEzJTI1JTNCJTIyJTNFJTNDYSUyMGNsYXNzJTNEJTIybW9yZSUyMiUyMGhyZWYlM0QlMjJodHRwJTNBJTJGJTJGdHJpYWx4LmNvbSUyRmN1cmV0YWxrJTJGMjAxOCUyRjA5JTJGMDclMkZsZXQtdGhlcmUtYmUtc2lnaHQtcmV2ZXJzaW5nLWJsaW5kbmVzcy13aXRoLXJldGluYWwtZ2VuZS10aGVyYXB5JTJGJTIyJTIwc3R5bGUlM0QlMjJjb2xvciUzQSUyM2ZmOTAwZSUyMCUyMWltcG9ydGFudCUzQiUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUzRVJlYWQlMjBtb3JlJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnAlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGFibGUlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGFibGUlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTNDJTJGdGFibGUlM0UlMEElM0MlMkZ0ZCUzRSUwQSUzQyUyRnRyJTNFJTBBJTNDdHIlM0UlMEElM0N0ZCUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGFsaWduJTNEJTIyY2VudGVyJTIyJTIwY2xhc3MlM0QlMjJsb2dvLXNvY2lhbCUyMiUzRSUwQSUyMCUyMCUzQ3RhYmxlJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGNlbGxwYWRkaW5nJTNEJTIyMCUyMiUyMGNlbGxzcGFjaW5nJTNEJTIyMCUyMiUyMHdpZHRoJTNEJTIyNjgwJTIyJTIwY2xhc3MlM0QlMjJlbWFpbENvbnRhaW5lci13aGl0ZSUyMG1haWwtY29udGFpbmVyJTIyJTIwc3R5bGUlM0QlMjJib3JkZXIlM0ExcHglMjBzb2xpZCUyMCUyM2YwZjBmMCUzQmJvcmRlci1ib3R0b20lM0EwcHglM0IlMjIlM0UlMEElMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJsZWZ0JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGFibGUlMjBib3JkZXIlM0QlMjIwJTIyJTIwY2VsbHBhZGRpbmclM0QlMjIyMCUyMiUyMGNlbGxzcGFjaW5nJTNEJTIyMCUyMiUyMHdpZHRoJTNEJTIyMTAwJTI1JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGFsaWduJTNEJTIybGVmdCUyMiUyMGNsYXNzJTNEJTIyY29sMi0xJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDcCUzRSUzQ2ElMjBocmVmJTNEJTIyaHR0cCUzQSUyRiUyRnRyaWFseC5jb20lMkYlMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlM0UlM0NpbWclMjBzcmMlM0QlMjJodHRwcyUzQSUyRiUyRmdhbGxlcnkubWFpbGNoaW1wLmNvbSUyRjE5ODhkMTE2YzQ0NDc1NjRjNThlMDNhNWUlMkZpbWFnZXMlMkY4YzM4YWJhZC00MWRmLTQ3MGItYTQwMi1iMTcwYzM0OTQ5ZWEucG5nJTIyJTIwYWx0JTNEJTIyJTIyJTIwYm9yZGVyJTNEJTIyMCUyMiUyMHN0eWxlJTNEJTIybWFyZ2luJTNBJTIwMCUzQiUyMHBhZGRpbmclM0ElMjAwJTNCJTIyJTIwbWMlM0FlZGl0JTNEJTIydHNpY29ubmltZzA3JTIyJTNFJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnAlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwYWxpZ24lM0QlMjJyaWdodCUyMiUyMGNsYXNzJTNEJTIyY29sMi0xJTIwbS1sZWZ0JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDcCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZ3d3cuZmFjZWJvb2suY29tJTJGdHJpYWx4JTJGJTIyJTIwdGFyZ2V0JTNEJTIyX2JsYW5rJTIyJTIwbWMlM0FlZGl0JTNEJTIydHNpY29ubmltZzA4JTIyJTNFJTNDaW1nJTIwc3JjJTNEJTIyaHR0cHMlM0ElMkYlMkZnYWxsZXJ5Lm1haWxjaGltcC5jb20lMkYxOTg4ZDExNmM0NDQ3NTY0YzU4ZTAzYTVlJTJGaW1hZ2VzJTJGNzllMDJlMWUtZWQwNS00M2Y1LTg2NzItMzNmNGYxOTQxMDZlLnBuZyUyMiUyMGFsdCUzRCUyMiUyMiUyMGJvcmRlciUzRCUyMjAlMjIlMjBzdHlsZSUzRCUyMm1hcmdpbiUzQSUyMDAlM0IlMjBwYWRkaW5nJTNBJTIwMCUzQiUyMiUzRSUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwaHJlZiUzRCUyMmh0dHBzJTNBJTJGJTJGdHdpdHRlci5jb20lMkZ0cmlhbHglMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlMjBtYyUzQWVkaXQlM0QlMjJ0c2ljb25uaW1nMDklMjIlM0UlM0NpbWclMjBzcmMlM0QlMjJodHRwcyUzQSUyRiUyRmdhbGxlcnkubWFpbGNoaW1wLmNvbSUyRjE5ODhkMTE2YzQ0NDc1NjRjNThlMDNhNWUlMkZpbWFnZXMlMkY0MGIyNjJhMC1iYTI5LTQ1NzAtODk5ZS05ODViYzFkMjk5NmYucG5nJTIyJTIwYWx0JTNEJTIyJTIyJTIwYm9yZGVyJTNEJTIyMCUyMiUyMHN0eWxlJTNEJTIybWFyZ2luJTNBJTIwMCUzQiUyMHBhZGRpbmclM0ElMjAwJTNCJTIyJTNFJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZ3d3cubGlua2VkaW4uY29tJTJGY29tcGFueSUyRnRyaWFseCUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUyMG1jJTNBZWRpdCUzRCUyMnRzaWNvbm5pbWcxMCUyMiUzRSUzQ2ltZyUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGZ2FsbGVyeS5tYWlsY2hpbXAuY29tJTJGMTk4OGQxMTZjNDQ0NzU2NGM1OGUwM2E1ZSUyRmltYWdlcyUyRjhjMDUzY2I5LTJiNWMtNDFmYy1hZTk0LWQ5MDdjMGE5NzI2Yy5wbmclMjIlMjBhbHQlM0QlMjIlMjIlMjBib3JkZXIlM0QlMjIwJTIyJTIwc3R5bGUlM0QlMjJtYXJnaW4lM0ElMjAwJTNCJTIwcGFkZGluZyUzQSUyMDAlM0IlMjIlM0UlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDYSUyMGhyZWYlM0QlMjJodHRwcyUzQSUyRiUyRnd3dy5pbnN0YWdyYW0uY29tJTJGdHJpYWx4Y29ubmVjdCUyRiUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUyMG1jJTNBZWRpdCUzRCUyMnRzaWNvbm5pbWcxMSUyMiUzRSUzQ2ltZyUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGZ2FsbGVyeS5tYWlsY2hpbXAuY29tJTJGMTk4OGQxMTZjNDQ0NzU2NGM1OGUwM2E1ZSUyRmltYWdlcyUyRjdmMzVlNDcyLWYwOTYtNDY1NC1iNmJhLTM4MDc2NDY2MGUyYS5wbmclMjIlMjBhbHQlM0QlMjIlMjIlMjBib3JkZXIlM0QlMjIwJTIyJTIwc3R5bGUlM0QlMjJtYXJnaW4lM0ElMjAwJTNCJTIwcGFkZGluZyUzQSUyMDAlM0IlMjIlM0UlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGcCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlM0MlMkZ0YWJsZSUzRSUwQSUyMCUyMCUzQyUyRnRkJTNFJTBBJTNDJTJGdHIlM0UlMEElM0MlMkZ0YWJsZSUzRSUwQSUzQyUyRnRkJTNFJTBBJTNDJTJGdHIlM0UlMEElM0N0ciUzRSUwQSUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJjZW50ZXIlMjIlMjBjbGFzcyUzRCUyMmVtYWlsLWZvb3RlciUyMiUzRSUwQSUzQ3RhYmxlJTIwYm9yZGVyJTNEJTIyMCUyMiUyMGNlbGxwYWRkaW5nJTNEJTIyMCUyMiUyMGNlbGxzcGFjaW5nJTNEJTIyMCUyMiUyMHdpZHRoJTNEJTIyNjgwJTIyJTIwY2xhc3MlM0QlMjJlbWFpbENvbnRhaW5lci1mb290ZXIlMjBtYWlsLWNvbnRhaW5lciUyMiUyMHN0eWxlJTNEJTIyYmFja2dyb3VuZCUzQSUyM2ZmOTAwZSUzQiUyMiUzRSUwQSUzQ3RyJTNFJTBBJTIwJTIwJTNDdGQlMjB2YWxpZ24lM0QlMjJ0b3AlMjIlMjBhbGlnbiUzRCUyMmxlZnQlMjIlM0UlMEElMjAlMjAlMjAlMjAlM0N0YWJsZSUyMGJvcmRlciUzRCUyMjAlMjIlMjBjZWxscGFkZGluZyUzRCUyMjIwJTIyJTIwY2VsbHNwYWNpbmclM0QlMjIwJTIyJTIwd2lkdGglM0QlMjIxMDAlMjUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwdmFsaWduJTNEJTIydG9wJTIyJTIwYWxpZ24lM0QlMjJsZWZ0JTIyJTIwY2xhc3MlM0QlMjJjb2wyLTElMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NwJTIwbWMlM0FlZGl0JTNEJTIyaWNvbm50ZXh0MjAlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NzbWFsbCUzRUNvcHlyaWdodCUyMCVDMiVBOSUyMDIwMTglMjBUcmlhbFguJTIwQWxsJTIwcmlnaHRzJTIwcmVzZXJ2ZWQuJTNDJTJGc21hbGwlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZwJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMHZhbGlnbiUzRCUyMnRvcCUyMiUyMGFsaWduJTNEJTIycmlnaHQlMjIlMjBjbGFzcyUzRCUyMmNvbDItMSUyMG0tbGVmdCUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3AlMjBtYyUzQWVkaXQlM0QlMjJpY29ubnRleHQyMSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NtYWxsJTNFJTNDYSUyMGhyZWYlM0QlMjIlMkElN0NBUkNISVZFJTdDJTJBJTIyJTNFVmlldyUyMG9ubGluZSUzQyUyRmElM0UlMjAlN0MlMjAlM0NhJTIwaHJlZiUzRCUyMiUyQSU3Q1VOU1VCJTdDJTJBJTIyJTNFVW5zdWJzY3JpYmUlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGc21hbGwlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZwJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUzQyUyRnRhYmxlJTNFJTBBJTNDJTJGdGQlM0UlMEElM0MlMkZ0ciUzRSUwQSUzQyUyRnRhYmxlJTNFJTBBJTNDJTJGdGQlM0UlMEElM0MlMkZ0ciUzRSUwQSUzQyUyRnRhYmxlJTNFJTBBJTNDJTJGYm9keSUzRSUwQSUzQyUyRmh0bWwlM0U=[/vc_raw_html][/vc_column][/vc_row]