Why is Participation of Healthy Volunteers in Clinical Trials Important?
Lack of volunteers is the reason behind 30% of clinical trials that never kickstart, and 20% of the trials that start, get aborted abruptly due to poor accrual.
TrialX iConnect is a portal that helps investigators expand the reach of their clinical trials to patients and hence help in patient recruitment. We are continuously adding new features to this portal to help investigators enhance their recruitment experience.
Currently iConnect is being used by institutions like Penn Medicine (UPENN), New York University (NYU), Beth Israel Deaconess Medical Center (BIDMC), Multiple Myeloma Research Foundation (MMRF) and New York Presbyterian for patient recruitment as well as by their patients for finding clinical trials.
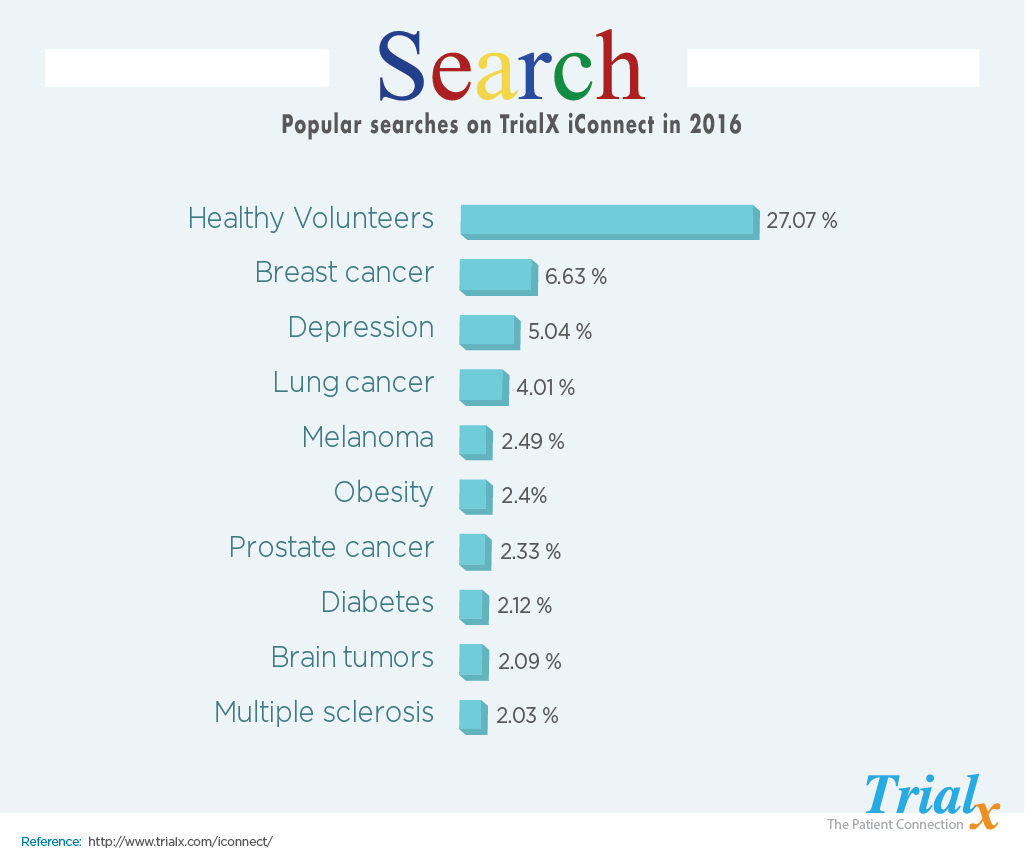
We did an analysis of all the terms searched by patients and investigators on these portals of iConnect in 2016. We found that “healthy volunteers” was the term that was searched maximum number of times followed by “breast cancer”, “depression”, “lung cancer”, and “melanoma” among top 5 searches. This data clearly shows the importance of participation of healthy volunteers in clinical trials.
Who is a healthy volunteer in a clinical Trial?
According to National Institute of Health (NIH) a healthy volunteer is “someone with no known significant health problems who participates in research to test a new drug, device, or intervention”. They can also be called “Clinical Research Volunteers”. Every year about 3500 healthy volunteers participate in clinical trials conducted by NIH.
Why are healthy volunteers needed?
Of the total terms that were searched on three iConnect portals, the term “healthy Volunteers” contributed to 27% of total terms searched across the 3 organizations.
Now, why are healthy participants needed? For any drug or device to get approved for marketing to general public, it needs to pass through 4 stages of human testing. Most of these clinical trials are designed to have a control arm and an experimental arm so as to get a comparison of effectiveness, toxicity and safety of a particular drug. In the experimental arm the intervention is the novel drug that is being tested. In the control arm the intervention is either standard treatment, or a control, or a placebo. The control arm of clinical trials mostly need healthy volunteers who provide data for comparison with people with illness.
Healthy volunteers may help the researchers define ”normal limits” in each of these phases of clinical trials.
Phase I – Test the safety of a drug and to understand its absorption, excretion and metabolism in human body. This phase usually needs healthy volunteers who does not have the disease that a particular drug or device is meant for.
Phase II – Tests the efficacy of a drug or device. These trials are mostly randomized, controlled trials with an experimental arm that receives the drug being tested and a control arm which receives either standard treatment or placebo. Some of the trials in this phase may need healthy volunteers for the control arm.
Phase III – Tests the effectiveness, benefits and range of effects more thoroughly using larger set of participants of about several hundreds to thousands including healthy volunteers. These trials are usually randomized, blinded experiments where the participants are not aware what treatment they are receiving. A pharmaceutical company can request FDA approval for marketing a drug or device, only after the completion of Phase III clinical trials.
Phase IV – Compares the efficacy, cost-effectiveness, and safety of a drug after it has been approved for consumer use. These trials may use healthy volunteers to study the effectiveness of a drug compared to a drug that is already on the market, to test its long-term effectiveness and cost effectiveness. These trials may result in a drug being withdrawn from the market or restrictions put on it, depending on the results.
Are there any eligibility criteria for healthy volunteers too?
Actually, there may be some. Healthy volunteers are often matched to patients so as to avoid variability in the research sample. Many studies match participants for characteristics like age, gender, smoking status or pregnancy status – depending on the study design.
Also your just feeling healthy may not be sufficient. The research team may get you tested for a few parameters to ensure that you belong to the “healthy” category before enrolling you. So, you may not be eligible to participate as a healthy volunteer in all the trials. It is always better to check with the clinical research coordinators or others on the clinical trial team about the tests you need to undergo or the past reports you need to show.
What do the healthy volunteers get in return?
It can be a win win situation for the healthy volunteers depending on the trials they choose to participate in. The healthy volunteers:
- Get a feeling of satisfaction of being the source of valuable scientific information for development of novel drugs and therapies.
- Have the satisfaction of being able to help others suffering from an illness.
- May get a thorough physical exam depending on the study.
- May receive compensation for participating.
- Get to increase their knowledge of clinical research and diseases.
Most of the times the healthy participants are paid for participating and also reimbursed for their time and travel.
What’s important to know before enrolling?
What’s important to know is that trials involving healthy volunteers are not necessarily always risk free. So if you are considering enrolling in one please read and understand the consent document carefully before signing it. The terminology used in these documents may be difficult to understand. Do not hesitate to ask for help from the research team to understand these.
Also it is advisable to clearly ask about the level of discomfort you might experience during the trial and any protection/treatment you are eligible to get in case there is an accident, including clarity on time and travel commitment. You can learn more about participating as a healthy volunteer by HERE.
Among the other top 5 searches on iConnect portals in 2016, “Depression” which was the theme for this World Health Day 2017 ranked third.
It is alarming to know that over 300 million people worldwide suffer from depression already, many ending their lives due to it. WHO predicts that the burden of mental disorders is expected to rise significantly over the next 20 years. Since, talking about it and discussing your problems is the best treatment of depression, WHO has started a one year campaign with the goal that more and more people with depression come out and seek help. You can access more information about the campaign HERE.