Diversity in Research Recruitment Management System Offers Insights about Equity

The lack of racial and ethnic diversity among clinical trial participants is a critical problem in medical research in the United States — a weakness exposed quite starkly by the COVID pandemic and the resulting national spotlight on 1) clinical trials and 2) healthcare inequity. How can we have the highest degree of confidence in the conclusions of our research if our research subject population is not representative of our national population? When it comes to safety and efficacy data, we cannot, really..
This is an issue we pay close attention to at TrialX. As a healthcare technology company we strive to innovate for impact. We are working to improve our technology and user experience as part of our commitment to improving diversity and inclusion in clinical research enrollment.
We began this initiative with an evaluation of our technology’s baseline performance in terms of serving historically underrepresented groups. We wanted to first know if our technology is demonstrating any net positive or negative outcomes. The results surprised us. The data from our iConnect patient recruitment management system at the University of Pennsylvania revealed a relatively high degree of diversity among members of the general public volunteering to be considered for clinical trials (without necessarily applying for specific trial opportunities). (Figure 1).
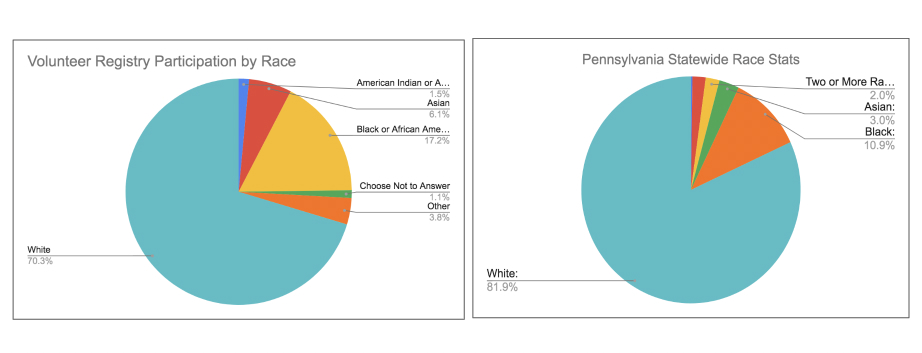
Whereas the statewide data from the most recent census places the overall population of black / African Americans at 11.17%, the percentage of iConnect volunteers who are black is more than 6 points higher, at 17.61%.
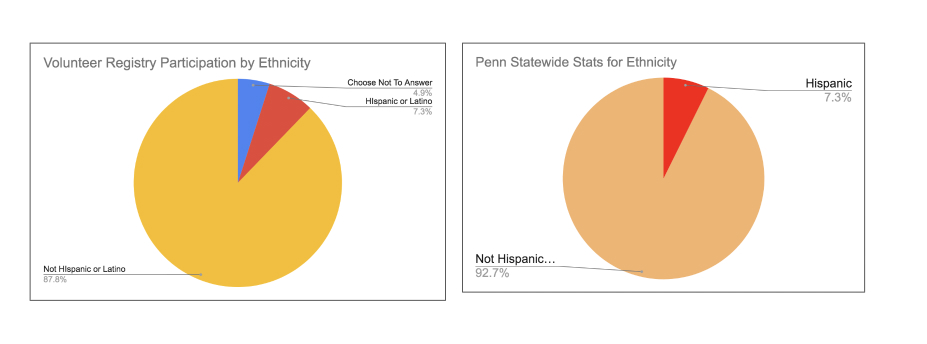
When measuring the level of interest among the different race groups we found that the numbers in Figure 2, below, offer even more cause for optimism about the potential for greater diversity in clinical research. This metric takes into account the race distribution of people who applied to participate in specific clinical trials found on iConnect.. As we saw in the Figure 1 table above, the overall distribution of race among volunteers has the black population at 17%. That total rises to 24.55% when we account for engagement measured by referral volumes across race. The data in the tables shows the same is true across other minority groups. This means that on our platform volunteers from minority populations are engaged and proactively pursuing participation in clinical research at higher rates than white participants.
As the public health enterprise ramps up efforts to improve diversity in research, our iConnect technology will be able to offer our enterprise clients the kind of insights that will drive things forward. Our ability to observe participation trends across racial boundaries will help us optimize our technology for greater equity for the underserved communities whose inclusion will be essential to achieve better health outcomes.


