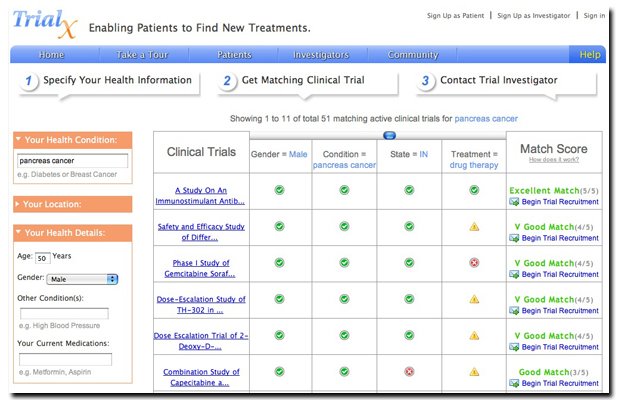
A 100% match
June 12th, 2008
•
195
From our discussions with other physicians, we have come to realize that it is very critical to achieve high specificity in the results – by that it means not to show a trial as matching trial even if it seems slightly relevant. We have been having discussions on whether showing a 100% match if the user has only entered on condition is appropriate? how about % of criteria that the entered condition matches?
Sure this will lower the match number significantly (and can potentially drive away some impatient users) but it will ensure that we are being highly specific (reducing false positives) and not unnecessarily raising hopes for a participant. We absolutely don’t want our users to take a print out and run down to their doctor downtown to learn that they did not meet an eligibility criteria.
We are working hard on processing as much of your medical information that we get and matching it automatically so you that can simply “Print & Ask Your Doctor!”
Categories:
information retrieval, matching, specificity
Tags:
#clinicalresearch ,
#digitalhealth ,
#MobiClin2018 ,
#scopesummit ,
15 years ,
2016 ,
ACRP2017 ,
active task ,
active task on ios ,
active tasks on android ,
Adventure ,
agentic ai ,
agentic ai in clinical trials ,
agentic ai in patient recruitment ,
AI ,
AI in Clinical Trials ,
AI in patient recruitment ,
AI-driven Patient recruitment tools ,
AICPA SOC 2 ,
ALS ,
Alzheimer's disease ,
American Heart Month ,
amia ,
AMIA 2016 ,
amia 2017 ,
AMIA2016 ,
AMIA2017 ,
amputee pain ,
Android ,
android apps ,
android research study apps ,
Android ResearchKit ,
app builder ,
app surveys ,
appbakery ,
apple active task ,
Apple ResearchKit ,
apps ,
Artificial Intelligence ,
artificial intelligence in clinical trials ,
ASCO 2017 ,
ask Dory ,
astronaut data ,
astronaut displays ,
astronaut health ,
astronaut interaction ,
astronaut safety ,
Ax-4 ,
Ax-4 Mission ,
axiom mission 4 ,
Axiom Space ,
barriers in mhealth ,
BCI in space ,
BigData ,
biomedical data collection ,
blood cancer awareness ,
brain health ,
brain initiative ,
brain-computer interfaces ,
breast cancer clinical trials ,
Cancer ,
cancer immunotherapy ,
Cancer in LEO-3 ,
cancer moonshot ,
cancer novartis ,
Cancer treatment ,
car t cancer ,
car t clinical trials ,
car t durgs ,
car t pharmas ,
CAR-T cell therapy ,
car-t immunotherapy ,
cardiovascular health ,
carekit ,
Carl June ,
Celiac disease ,
cellectar ,
centerwatch ,
cervical cancer awareness ,
cervical cancer clinical trials ,
cervical health ,
challenges mhealth adoption ,
chatbots ,
clinical ,
clinical research ,
clinical trial ,
clinical trial awareness ,
clinical trial digital tools ,
clinical trial failure ,
clinical trial finder ,
clinical trial losses ,
clinical trial management ,
clinical trial management system ,
clinical trial management systems ,
clinical trial participation ,
clinical trial software ,
clinical trial sponsors ,
clinical trial website builder ,
clinical trials ,
clinical trials eligibility criteria ,
Clinical Trials in Space ,
clinical trials participation ,
clinical trials recruitment ,
clinical trials upenn ,
clinicalresearch ,
clinicaltrials ,
clinicaltrialvolunteers ,
commercial astronauts ,
Company Retreat ,
conference 2016 ,
conference 2017 ,
conference 2023 ,
consent under gdpr ,
Coronavirus ,
covid talk series with Penn Medicine ,
covid trials ,
COVID-19 ,
COVID-19 Cure ,
COVID19 ,
covid19 clinical trials ,
COVID19 therapy ,
COVID19 vaccine ,
Creative writing ,
crew health monitoring ,
crossplatform apps ,
ctms ,
ctms trialx ,
Cure ,
curetalks@penn ,
data accuracy ,
data privacy ,
data quality ,
DCT ,
Decentralised clinical trials ,
decentralized clinical trial ,
decentralized research ,
decentralized trials ,
DEI Toolkit ,
depression ,
depression clinical trials ,
depression research ,
DIA ,
DIA 2016 ,
DIA2017 ,
diabetes clinical trials ,
digital patient engagement ,
disruptive innovations ,
diversity ,
diversity in clinical trials ,
DIY appmaking platform ,
DIY study apps ,
dpharm ,
DPharm 2016 ,
Dpharm2016 ,
dpharm2017 ,
drug development ,
drugs in 2016 ,
e-consent ,
eCOA ,
EHR ,
electronic Clinical Outcome Assessments ,
electronic displays in space ,
electronic patient reported outcomes ,
ePRO ,
equity ,
equity in clinical trials ,
EVA and Spacesuits ,
EXPAND database ,
extreme environment remote data collection ,
facebook ,
fda ,
FDA approvals ,
fda drug approval process ,
fda drug approved in 2016 ,
FDA perspective ,
Federal data ,
finger tapping researchkit ,
fNIRS ,
games ,
gamification ,
GDPR ,
genome hacking ,
global clinical trials ,
gregory dumanian ,
Hackathon ,
Halloween ,
healthcare ,
healthy controls ,
healthy subjects ,
healthy volunteers ,
HERMES platform ,
HIPAA compliance ,
human atlas ,
human cell atlas ,
human factors in space ,
iconnect ,
iconnect clinical trial ,
iConnect features ,
IDY ,
Immunotherapy ,
immunotherapy cancer ,
inclusion ,
inclusion in clinical trials ,
indiana clinical trials ,
informatics ,
informed consent ,
Inspiration ,
international space station ,
internationalization in research study apps ,
investment ,
iOS ,
ios apps ,
ISRO Space Research ,
key fda drugs ,
limb loss pain ,
lung cancer awareness ,
Lyme ,
lyme diagnosis ,
Lyme disease ,
lyme disease management ,
Lyme disease tracker app ,
lyme symptoms ,
medical apps ,
medical breakthrough ,
medical imaging ,
Mega projects ,
mental health ,
mental health clinical trials ,
mhealth ,
mhealth adoption ,
mhealth in clinical trials ,
mhealth precision medicine ,
mhealth tools ,
microgravity ,
Microgravity Effects on Muscles ,
microgravity research ,
milestone ,
minority participation ,
Mitochondrial Function in Space ,
mobile apps ,
mobile clinical innovations ,
mobile health technology in engagment ,
mobile in clinical trials ,
Mobile Research ,
Mobile Research Apps ,
mobile research study app ,
mobile research study apps ,
mobileclin2017 ,
mobileclin2022 ,
mrna ,
mrna vaccine ,
multi-language apps ,
multi-language research apps ,
Muscle Loss in Space ,
my clinical trial story ,
Myogenesis Experiment ,
NASA spacesuit audit ,
neurogenic pain ,
new data privacy policy ,
new release ,
new video format ,
nobel prize ,
nobel prize laureates ,
northwestern university ,
novel coronavirus ,
NYU ,
nyu clinical trials ,
online talk shows ,
orphan drugs ,
ovarian cancer awareness ,
pain app ,
pancreatic cancer clinical trials ,
pancreatic cancer research studies ,
patient ,
patient centric ,
patient centricity ,
patient engagement ,
patient engagement in clinical trials ,
patient generated health data ,
patient involvement ,
patient involvement in clinical trials ,
patient participation ,
patient portals ,
Patient Recruitment ,
patient referrals ,
patient reported outcomes ,
patient safety ,
patient-reported outcomes ,
patientengagement ,
patientfocussed ,
patients ,
patienty privacy ,
penn clinical trials ,
Penn Medicine ,
personal health devices ,
personal health information gdpr ,
Philadelphia ,
physiology and medicine ,
precision medicine ,
privacy and security ,
prompts ,
rare disease ,
rare disease awareness ,
rare disease day ,
real-time data ,
Recruitment Tools ,
regulatory compliance ,
remote data ,
remote data collection ,
remote diagnostics ,
Remote Health Data Collection ,
Remote Research Data Collection ,
remote research data collection platform ,
research ,
research apps ,
research study apps ,
Research Volunteers ,
ResearchDroid ,
researchkit ,
researchkit active task ,
researchkit active task list ,
researchkit active tasks ,
researchkit android ,
researchkit apps ,
rett syndrome trials ,
Sars-Cov-2 ,
SARS-COV2 ,
SCOPE2018 ,
scope2023 ,
SCOPE2025 ,
scopesummit ,
security compliance ,
sellas life sciences ,
sickle cell disease ,
sleep in space ,
sleep self management ,
sleep study ,
sleep tracking ,
sleep tracking app ,
small biotech companies ,
SOC 2 ,
SOC 2 Compliance ,
Social Media ,
space clinical research ,
Space Health ,
space health research ,
space health technology ,
space medicine ,
space neuroscience ,
space oncology ,
space research ,
spaceflights ,
spacehealth ,
spaceresearch ,
Stem Cell Regeneration ,
study apps ,
Team building ,
Team Outing ,
Team Retreat ,
Telemetric Health AI ,
The Opportunity Project ,
thyroid awareness month ,
tokenization ,
TOP Health Challenge ,
tophealth ,
tophealthchallenge ,
track sleep ,
trial participation and engagment ,
trialspace ,
trialx ,
trialx iconnect ,
TrialX Space Health Systems ,
triple-negative breast cancer ,
trump fda ,
tsc ,
tuberous sclerosis complex ,
twitter ,
U.S. Elections ,
ultrasound ,
University of Pennsylvania ,
unsung but impactful ,
Upenn ,
Virology ,
voices of clinical research ,
volunteer registry ,
wearable devices ,
wearables ,
Webinars ,
Website Builder ,
weight loss clinical trials ,
weight management clinical trials ,
White House ,
world cancer day ,
world yoga day ,
year end review ,
year2022 ,
3
24637