Key FDA Approved Drugs in 2016
While President Trump believes that ‘slow and burdensome’ FDA drug approval process is ‘keeping too many new drugs from reaching the market’, former FDA Director Rob Califf differs in his opinion when he says that ‘Drug regulation drives innovation’.
Rob Califf, a cardiologist goes on to elaborate, (in Forbes), that the drug development and approval process being followed is to save situations like that of birth defects caused by thalidomide. In order to approve a drug, the FDA has to assure that the drug/medical device is completely safe for use by humans. The safety and efficacy terms and conditions enforced by the FDA for drug approval forces pharmaceutical companies to bring in innovation as well as improvements to existing therapies.
Let us take a look at the current FDA approval process:
It takes 12 years on an average and over US$350 million to get a new drug from the laboratory onto the pharmacy shelf. Once a company develops a drug, it undergoes around three and a half years of laboratory testing, before an application is made to the U.S. Food and Drug Administration (FDA) to begin testing the drug in humans. Only one in 1000 of the compounds that enter laboratory testing will ever make it to human testing.
If the FDA gives the green light, the “investigative” drug will then enter three phases of clinical trials:
- Phase 1 uses 20-80 healthy volunteers to establish a drug’s safety and profile. (about 1 year)
- Phase 2 employs 100-300 patient volunteers to assess the drug’s effectiveness. (about 2 years)
- Phase 3 involves 1000-3000 patients in clinics and hospitals who are monitored carefully to determine effectiveness and identify adverse reactions. (about 3 years)
The company then submits an application to the FDA for approval, a process that can take up to two and a half years. The FDA reviews a standard drug application within 12 months and a priority application within 6 months. Application for drugs similar to those on the market are considered standard, whereas priority applications represent drugs offering important advances in addition to existing treatments. After final approval, the drug becomes available for physicians to prescribe. At this stage, the drug company will continue to report cases of adverse reactions and other clinical data to the FDA.
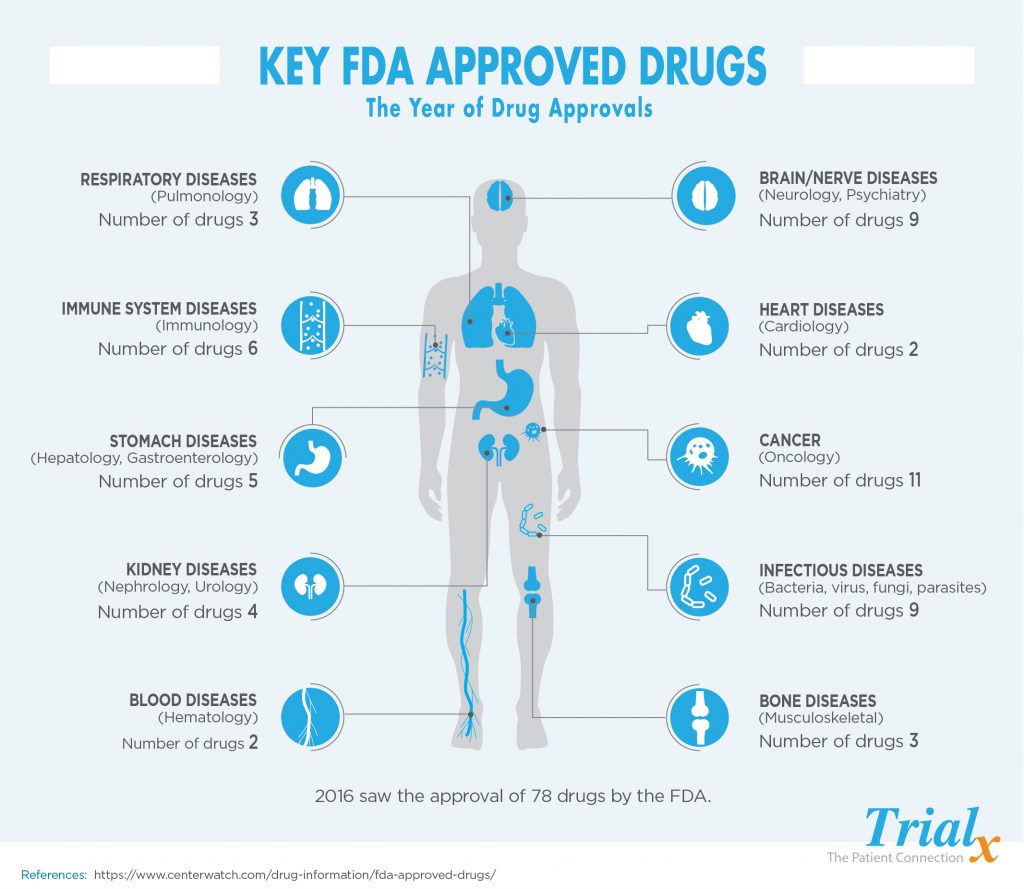
The year 2016 turned out to be a disappointing one for NEW drug approvals with the U.S. Food and Drug Administration clearing just 22 new medicines for sale. The annual report from the FDA’s Office of New Drugs provides some insights into the sharp drop-off. ( “new” drug approvals refer to new therapies, and wouldn’t include, for example, an existing cancer drug that was already approved to treat melanoma and then gained an additional indication to treat lung cancer.)
Here is a look at the drugs approved by the FDA in 2016:
Cardiology/Vascular Diseases
- Byvalson (nebivolol and valsartan); Allergan; For the treatment of hypertension, Approved June 2016
- Yosprala (aspirin and omeprazole); Aralez Pharmaceuticals; For the prevention of cardiovascular and cerebrovascular events, Approved September 2016
Dermatology
- Ameluz (aminolevulinic acid hydrochloride); Biofrontera Pharma; For the treatment of actinic keratoses, Approved May 2016
- Eucrisa (crisaborole) ointment; Pfizer; For the treatment of atopic dermatitis, Approved December 2016
- Taltz (ixekizumab); Eli Lilly; For the treatment of plaque psoriasis, Approved March 2016
Endocrinology
- Adlyxin (lixisenatide); Sanofi Aventis; For the treatment of type II diabetes, Approved July 2016
- Soliqua 100/33 (insulin glargine and lixisenatide injection); Sanofi Aventis; For the treatment of inadequately controlled type II diabetes, Approved November 2016
- Xultophy 100/3.6 (insulin degludec and liraglutide injection); Novo Nordisk; For the treatment of inadequately controlled type II diabetes, Approved November 2016
Family Medicine
- Byvalson (nebivolol and valsartan); Allergan; For the treatment of hypertension, Approved June 2016
- Onzetra Xsail (sumatriptan nasal powder) ; Avanir; For the treatment of migraine, Approved January 2016
- Soliqua 100/33 (insulin glargine and lixisenatide injection); Sanofi Aventis; For the treatment of inadequately controlled type II diabetes, Approved November 2016
- Xultophy 100/3.6 (insulin degludec and liraglutide injection); Novo Nordisk; For the treatment of inadequately controlled type II diabetes, Approved November 2016
Gastroenterology
- Zinplava (bezlotoxumab); Merck; For the treatment of recurrent Clostridium difficile infection in patients receiving antibacterial treatment, Approved October 2016
Genetic Disease
- Spinraza (nusinersen); Biogen; For the treatment of spinal muscular atrophy, Approved December 2016
Hematology
- Afstyla (Antihemophilic Factor (Recombinant), Single Chain); CSL Behring; For the treatment of hemophillia A, Approved May 2016
- Idelvion (Coagulation Factor IX (Recombinant), Albumin Fusion Protein); CSL Behring; For the treatment of hemophilia B, Approved March 2016
- Kovaltry [Antihemophilic Factor (Recombinant)]; Bayer ; For the treatment of hemophillia A, Approved March 2016
- Opdivo (nivolumab); Bristol-Myers Squibb; For the treatment of classical Hodgkin lymphoma, Approved May 2016
- Venclexta (venetoclax); AbbVie; For the treatment of chronic lymphocytic leukemia with 17p deletion, Approved April 2016
Hepatology (Liver, Pancreatic, Gall Bladder)
- Defitelio (defibrotide sodium); Jazz Pharmaceuticals; For the treatment of hepatic veno-occlusive disease with renal or pulmonary dysfunction following HSCT, Approved March 2016
- Ocaliva (obeticholic acid); Intercept Pharmaceuticals; For the treatment of primary biliary cholangitis, Approved May 2016
- Vemlidy (tenofovir alafenamide); Gilead Sciences; For the treatment of chronic hepatitis B , Approved November 2016
- Zepatier (elbasvir and grazoprevir); Merck; For the treatment of chronic HCV genotypes 1 or 4 , Approved January 2016
Immunology
- Afstyla (Antihemophilic Factor (Recombinant), Single Chain); CSL Behring; For the treatment of hemophillia A, Approved May 2016
- Descovy (emtricitabine and tenofovir alafenamide); Gilead; For the treatment of HIV-1 infection, Approved April 2016
- Epclusa (sofosbuvir and velpatasvir) ; Gilead Sciences; For the treatment of hepatitis C, Approved June 2016
- Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide); Gilead Sciences; For the treatment of HIV-1 as initial therapy, Approved March 2016
- Taltz (ixekizumab); Eli Lilly; For the treatment of plaque psoriasis, Approved March 2016
- Vaxchora (Cholera Vaccine, Live, Oral); PaxVax; For active immunization against Cholera, Approved June 2016
Infections and Infectious Diseases
- Anthim (obiltoxaximab); Elusys Therapeutics; For the treatment of inhalational anthrax , Approved March 2016
- Descovy (emtricitabine and tenofovir alafenamide); Gilead; For the treatment of HIV-1 infection, Approved April 2016
- Epclusa (sofosbuvir and velpatasvir) ; Gilead Sciences; For the treatment of hepatitis C, Approved June 2016
- Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide); Gilead Sciences; For the treatment of HIV-1 as initial therapy, Approved March 2016
- Syndros (dronabinol oral solution); Insys Therapeutics; For the treatment of anorexia associated with AIDS and nausea and vomiting associated with cancer chemotherapy, Approved July 2016
- Vaxchora (Cholera Vaccine, Live, Oral); PaxVax; For active immunization against Cholera, Approved June 2016
- Vemlidy (tenofovir alafenamide); Gilead Sciences; For the treatment of chronic hepatitis B , Approved November 2016
- Zepatier (elbasvir and grazoprevir); Merck; For the treatment of chronic HCV genotypes 1 or 4 , Approved January 2016
- Zinplava (bezlotoxumab); Merck; For the treatment of recurrent Clostridium difficile infection in patients receiving antibacterial treatment, Approved October 2016
Musculoskeletal
- Exondys 51 (eteplirsen); Sarepta Therapeutics; For the treatment of Duchenne muscular dystrophy with mutated DMD gene amenable to exon 51 skipping, Approved September 2016
- Spinraza (nusinersen); Biogen; For the treatment of spinal muscular atrophy, Approved December 2016
- Zinbryta (daclizumab) ; Biogen; For the treatment of relapsing multiple sclerosis, Approved May 2016
Nephrology
- Cabometyx (cabozantinib); Exelixis; For the treatment of advanced renal cell carcinoma, Approved April 2016
- Lenvima (lenvatinib); Eisai; For the treatment of advanced renal cell carcinoma, Approved May 2016
- Rayaldee (calcifediol) ; Opko Health; For the treatment of secondary hyperparathyroidism in adults with stage 3 or 4 chronic kidney disease, Approved June 2016
Neurology
- Briviact (brivaracetam); UCB; For the treatment of partial onset seizures related to epilepsy, Approved February 2016
- Carnexiv (carbamazepine); Lundbeck; replacement therapy when oral administration is not feasible, in adults with seizures, Approved October 2016
- Exondys 51 (eteplirsen); Sarepta Therapeutics; For the treatment of Duchenne muscular dystrophy with mutated DMD gene amenable to exon 51 skipping, Approved September 2016
- Nuplazid (pimavanserin); Acadia Pharmaceuticals; For the treatment of hallucinations and delusions associated with Parkinson’s disease, Approved April 2016
- Nuplazid (pimavanserin); Acadia Pharmaceuticals; For the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, Approved May 2016
- Onzetra Xsail (sumatriptan nasal powder) ; Avanir; For the treatment of migraine, Approved January 2016
- Spinraza (nusinersen); Biogen; For the treatment of spinal muscular atrophy, Approved December 2016
- Troxyca ER (oxycodone + naltrexone); Pfizer; For the management of severe pain, Approved August 2016
- Zinbryta (daclizumab) ; Biogen; For the treatment of relapsing multiple sclerosis, Approved May 2016
Obstetrics/Gynecology (Women’s Health)
- Intrarosa (prasterone vaginal insert); Endoceutics; For the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause, Approved November 2016
- Rubraca (rucaparib); Clovis Oncology; For the treatment of advanced ovarian cancer in women with deleterious germline or somatic BRCA mutation, Approved December 2016
Oncology
- Cabometyx (cabozantinib); Exelixis; For the treatment of advanced renal cell carcinoma, Approved April 2016
- Keytruda (pembrolizumab); Merck; For the treatment of head and neck squamous cell cancer , Approved August 2016
- Lartruvo (olaratumab) ; Eli Lilly; For the treatment of soft tissue sarcoma, Approved October 2016
- Lenvima (lenvatinib); Eisai; For the treatment of advanced renal cell carcinoma, Approved May 2016
- Opdivo (nivolumab); Bristol-Myers Squibb; For the treatment of classical Hodgkin lymphoma, Approved May 2016
- Opdivo (nivolumab); Bristol-Myers Squibb; For the treatment of recurrent or metastatic squamous cell carcinoma of the head and neck, Approved November 2016
- Rubraca (rucaparib); Clovis Oncology; For the treatment of advanced ovarian cancer in women with deleterious germline or somatic BRCA mutation, Approved December 2016
- Sustol (granisetron); Heron Therapeutics; For the prevention of chemotherapy-induced nausea and vomiting, Approved August 2016
- Syndros (dronabinol oral solution); Insys Therapeutics; For the treatment of anorexia associated with AIDS and nausea and vomiting associated with cancer chemotherapy, Approved July 2016
- Tecentriq (atezolizumab); Genentech; For the treatment of urothelial carcinoma and metastatic non-small cell lung cancer, Approved May 2016
- Venclexta (venetoclax); AbbVie; For the treatment of chronic lymphocytic leukemia with 17p deletion, Approved April 2016
Ophthalmology
- Humira (adalimumab); Abbvie; For the treatment of uveitis, Approved July 2016
- Xiidra (lifitegrast); Shire; For the treatment of dry eye disease, Approved July 2016
Pediatrics/Neonatology
- Exondys 51 (eteplirsen); Sarepta Therapeutics; For the treatment of Duchenne muscular dystrophy with mutated DMD gene amenable to exon 51 skipping, Approved September 2016
- Kovaltry [Antihemophilic Factor (Recombinant)]; Bayer ; For the treatment of hemophillia A, Approved March 2016
- Spinraza (nusinersen); Biogen; For the treatment of spinal muscular atrophy, Approved December 2016
Pharmacology/Toxicology
- Sustol (granisetron); Heron Therapeutics; For the prevention of chemotherapy-induced nausea and vomiting, Approved August 2016
Psychiatry/Psychology
- Nuplazid (pimavanserin); Acadia Pharmaceuticals; For the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, Approved May 2016
Pulmonary/Respiratory Diseases
- Bevespi Aerosphere (glycopyrrolate and formoterol fumarate); AstraZeneca; For the treatment of chronic obstructive pulmonary disease, Approved April 2016
- Cinqair (reslizumab); Teva Pharmaceuticals; For the treatment of severe asthma, Approved March 2016
- Tecentriq (atezolizumab); Genentech; For the treatment of urothelial carcinoma and metastatic non-small cell lung cancer, Approved May 2016
Urology
- Tecentriq (atezolizumab); Genentech; For the treatment of urothelial carcinoma and metastatic non-small cell lung cancer, Approved May 2016
Vaccines
- Vaxchora (Cholera Vaccine, Live, Oral); PaxVax; For active immunization against Cholera, Approved June 2016
According to Vox, ‘the FDA is the fastest drug regulatory agency in the world’. The FDA also has many exceptions where drugs can be approved fairly faster like accelerated approvals, orphan drugs, priority preview and fast track.
However, whether relaxing the drug approval process will provide improved treatment options for patients remain to be seen.