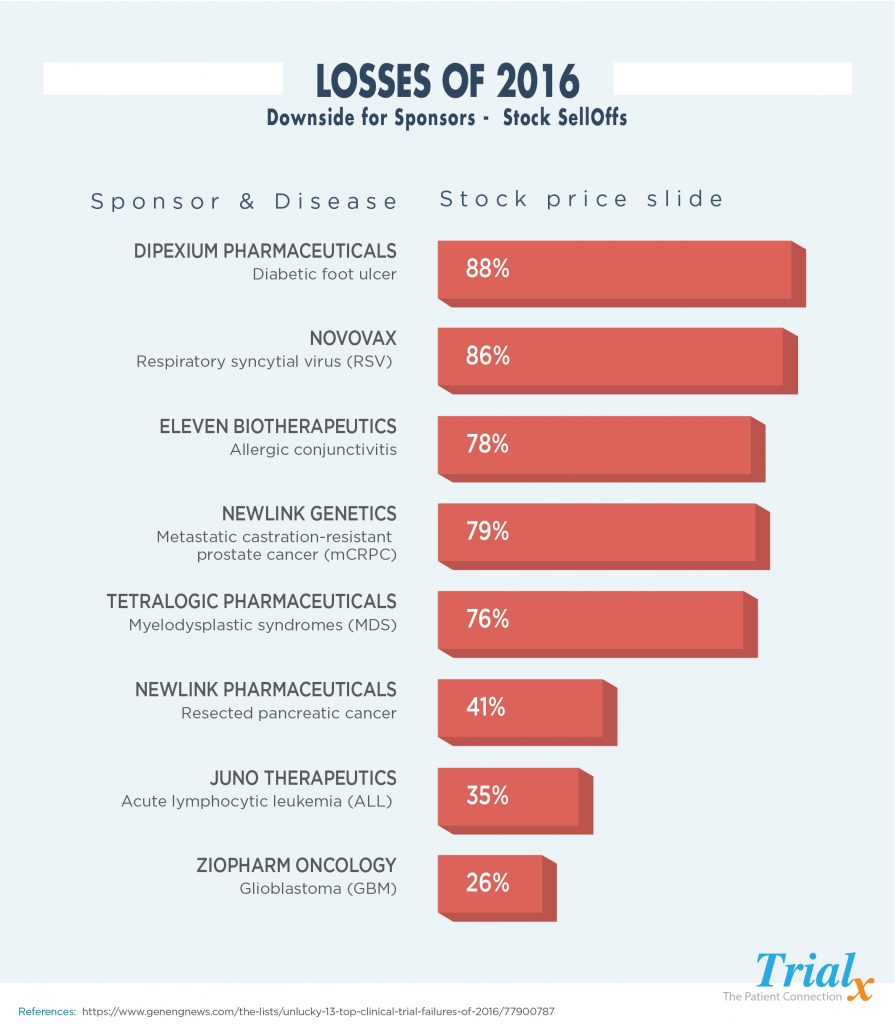
Clinical Trial Failures – Losses of 2016
Drug development costs are increasing and the time it takes to bring a drug to market is becoming longer. Also of consideration is the success rate for receiving regulatory approval to market your drug or device.The increasing cost of clinical research has significant implications for public health, as it affects drug companies’ willingness to undertake clinical trials, which in turn limits patient access to novel treatments.
A recently published study on pharmaceutical and biotechnology industry, outlines, clinical trial costs as below:
- Phase 1 study cost from US$1.4 million to US$6.6 million, including estimated site overhead and monitoring costs of the sponsoring organization.
- Phase 2 study cost from US$7.0 million to US$19.6 million
- Phase 3 study cost ranged from US$11.5 million to US$52.9 million on average.
Across all study phases and excluding estimated site overhead costs and costs for sponsors to monitor the study, the top three cost drivers of clinical trial expenditures were,
- Clinical procedure costs (15%–22% of total)
- Administrative staff costs (11%–29% of total)
- Site monitoring costs (9%–14% of total)
So, then what happens when some of the trials have to be terminated – layoffs, company stock sell outs and closures. Let us take a look at some of the clinical trials that ended in great losses for the sponsors in the year 2016:
A study in Nature Biotech in 2014 showed that only 32% of drugs have a probability of making it to Phase 3 trials, and only one in 10 drugs overall actually makes it to market. Things haven’t improved since then.
The cost of failed clinical trials is high, and the industry needs to focus on ways to reduce the continuously high failure rate. Cost estimates of failed trials range anywhere from $800 million to $1.4billion. Companies that experience failed trials often face a plummeting stock price and need to reduce workforce, close research sites and consolidate business units, and potentially sell off various therapeutic areas in order to preserve the core business. This of course impacts employees and shareholders, but even more so patients.
Why do clinical trials fail?
At first glance, one could conclude that the reason is the stringent FDA review process designed to ensure that only products that deliver an acceptable efficacy and safety profile ever reach consumers. But a more detailed analysis of the results of many clinical research programs tell a different story.
- Lack of efficacy
- Failure to maintain proper manufacturing protocols
- Inability to meet predetermined criteria and timelines set by the FDA
- Failing to properly interpret FDA feedback and interactions
- Misrepresenting a drug’s safety profile by not familiarizing the FDA with the full scope of any potential adverse events before the review;
- Insufficient proof of concept data; and,
- Trial designs that are inconsistent with clinical endpoints.
According to a recent study of 842 molecules and 637 development program failures, companies that take steps to identify potential problems in advance and then proactively stop development of an imperiled trial have a much higher likelihood of ultimately reaching the market with their drugs. Finding flaws in the development protocol can allow many companies to take the necessary steps to eliminate or reduce risk and make informed decisions about whether or not to shut down the trial.
Clinical trial costs are up but there are areas and ways to manage and even decrease them. This means considering all the options that are available today and collaborating with experienced and progressive partners. There is a realization that the process is evolving and technology is changing the landscape forever.