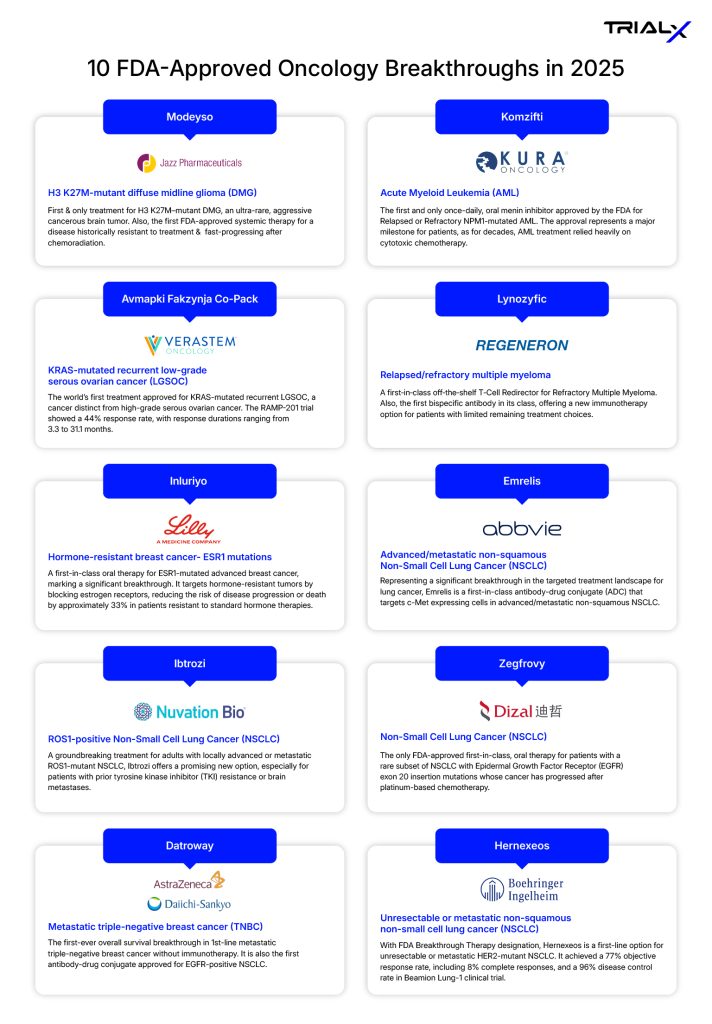
World Cancer Day: 10 FDA-Approved Oncology Breakthrough Drugs of 2025 That Marked Major Advances in Cancer Care

Each year, nearly 20 million people worldwide are diagnosed with cancer, a number projected to rise sharply in the coming decades. For patients and families, a diagnosis often brings uncertainty, difficult treatment decisions, and limited options—particularly for rare, aggressive, or treatment-resistant cancers.
World Cancer Day is a moment to acknowledge this global burden—but also to recognize the progress that continues to reshape cancer care. In 2025, the FDA approved several oncology therapies that represent meaningful advances, especially for cancers where few effective treatments previously existed. From targeted therapies and antibody–drug conjugates to next-generation immunotherapies, these approvals are expanding options and improving outcomes across multiple cancer types.
In this blog, we take a closer look at some of the most impactful cancer drug approvals of 2025.
10 FDA-Approved Oncology Breakthroughs in 2025
- Modeyso (dordaviprone)
Sponsor- Jazz Pharmaceuticals
Approved as the first and only treatment for recurrent H3K27M-mutant diffuse midline glioma (DMG)—a rare and aggressive brain tumor that primarily affects children and young adults—this therapy marks a significant milestone in the treatment landscape for one of the most devastating diagnoses.
The approval was based on pooled data from 50 patients, which showed a 22% overall response rate and a median response duration of 10.3 months. As the first systemic therapy approved for H3 K27M–mutant diffuse midline glioma, Modeyso (previously known as ONC-201) represents an important breakthrough in neuro-oncology, offering a long-awaited option where none previously existed.
- Komzifti (ziftomenib)
Sponsor- Kuro Oncology
NPM1-mutated acute myeloid leukemia (AML) is a cancer of the blood and bone marrow caused by a genetic change in the NPM1 gene, which is found in about 30% of AML cases. This mutation drives the rapid production of abnormal white blood cells, crowding out healthy cells and limiting the bone marrow’s ability to produce red blood cells and platelets. While this form of AML occurs primarily in adults, it can also affect children.
Because the NPM1 mutation plays a key role in how the disease develops, it has become an important target for new therapies. Komzifti, the first and only once-daily oral menin inhibitor, is approved for adults with relapsed or refractory AML with a susceptible NPM1 mutation. Taken as a once-daily pill, it offers a new option for patients whose disease has returned or stopped responding to treatment, addressing an area of long-standing unmet need.
- Avmapki Fakzynja Co-Pack (avutometinib + defactinib)
Sponsor- Verastem Oncology
Low-grade serous ovarian cancer (LGSOC) is a rare and slow-growing subtype of ovarian cancer that behaves differently from the more common high-grade form and often responds poorly to standard chemotherapy. Many cases are driven by genetic alterations such as KRAS mutations, leaving patients with limited targeted treatment options when the disease recurs.
Avmapki Fakzynja Co-Pack, developed by Verastem Oncology, is the first FDA-approved treatment for patients with recurrent LGSOC with a KRAS mutation. This approval marks a major milestone for a cancer that has long lacked effective, targeted therapies. The FDA’s decision was based on results from the Phase 2 RAMP 201 trial, which demonstrated a 44% overall response rate, supporting the therapy’s potential to address a significant unmet need in KRAS-mutated recurrent LGSOC.
A study evaluating avutometinib and defactinib in people with LGSOC is ongoing—learn more here.
- Lynozyfic (linvoseltamab-gcpt)
Sponsor- Regeneron
Lynozyfic represents a new generation of precision immunotherapy for multiple myeloma. It is a bispecific antibody, designed to bind both cancer cells and T cells at the same time, physically bringing the immune system into close contact with the tumor. This “immune-redirecting” approach helps the body’s own defenses recognize and kill myeloma cells more effectively. For patients with relapsed or refractory disease who have exhausted multiple treatment options, Lynozyfic has shown promising results. In the Phase 1/2 LINKER-MM1 trial, patients achieved a 70% overall response rate, with 45% reaching a complete response or better. Responses occurred rapidly, with a median time to response of just 0.95 months, and were durable, with most responders maintaining benefit beyond 9–12 months.
- Inluriyo (imlunestrant)
Sponsor: Eli Lilly and Company
Inluriyo is FDA-approved as an oral selective estrogen receptor degrader (SERD) for adults with ESR1-mutated, ER+, HER2– advanced or metastatic breast cancer. ESR1 mutations occur in an estimated 50% of patients who progress on aromatase inhibitors and often drive resistance to standard endocrine therapies. In the Phase 3 EMBER-3 trial, Inluriyo reduced the risk of progression or death by 38% compared with standard endocrine therapy. Its once-daily oral dosing allows patients to take treatment at home, offering both convenience and a meaningful new option for managing ESR1-mutated breast cancer.
A study is ongoing to evaluate imlunestrant in early ER+, HER2‑ breast cancer—learn more here.
- Emrelis (telisotuzumab vedotin-tllv)
Sponsor- AbbVie
Emrelis represents a meaningful step forward in the treatment of lung cancer, particularly for patients with limited targeted options. As a first-in-class antibody–drug conjugate (ADC), it is designed to seek out c-Met–expressing cancer cells and deliver a potent microtubule inhibitor directly to the tumor, combining precision targeting with strong anti-cancer activity. Administered via infusion, Emrelis is the first therapy specifically approved for this patient group, with ongoing studies expected to further validate its clinical benefit.
This advancement is especially significant given the burden of lung cancer, which remains the leading cause of cancer-related deaths worldwide. Nearly 85% of lung cancers are classified as non-small cell lung cancer (NSCLC), and despite recent therapeutic progress, many patients continue to face poor outcomes—underscoring the importance of targeted innovations like c-Met–directed ADCs that aim to improve precision and effectiveness in treatment.
- Ibtrozi (taletrectinib)
Sponsor- Nuvation Bio
Ibtrozi represents an important advance for patients with ROS1-positive non-small cell lung cancer (NSCLC), particularly those with brain involvement or treatment resistance. The drug is designed to overcome resistance mutations, demonstrates strong activity against brain metastases, and avoids the neurological side effects seen with some existing therapies—a critical benefit given that nearly 35% of patients with metastatic ROS1-positive NSCLC have brain metastases at diagnosis.
Its approval is supported by robust Phase 3 evidence from the TRUST-1 and TRUST-2 trials. When used as a first-line treatment, Ibtrozi achieved objective response rates of 90% and 84%, respectively, as reported at last year’s ESMO Congress. Notably, the drug also showed meaningful efficacy in previously treated patients, with response rates ranging from 52% to 62%, reinforcing its potential role across multiple lines of therapy.
- Zegfrovy (sunvozertinib)
Sponsor- Dizal Pharmaceuticals
ZEGFROVY is the only approved targeted oral treatment for non-small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations, a subtype known for its poor prognosis and limited treatment options. Patients with these mutations typically experience shorter survival—often less than 18 months—compared with those who have more common EGFR alterations.
The drug received accelerated FDA approval following Priority Review, supported by results from the pivotal WU-KONG1 Part B study. In this trial, ZEGFROVY delivered statistically significant and clinically meaningful benefits, achieving an overall response rate of 46% and a median duration of response of 11.1 months. The approval applies to patients whose disease has progressed on or after platinum-based chemotherapy, addressing a critical unmet need in this hard-to-treat population.
Several clinical trials are ongoing to evaluate sunvozertinib —check active trials here.
- Datroway (datopotamab deruxtecan-dlnk)
Sponsor- AstraZeneca
Datroway is the first antibody–drug conjugate approved for EGFR-positive non-small cell lung cancer (NSCLC). It targets cancer cells via a protein called TROP2 on their surface, delivering potent anti-cancer medicine directly into tumor cells while sparing healthy tissue.
In breast cancer, the TROPION-Breast02 trial demonstrated an overall survival benefit in the first-line treatment of patients with metastatic triple-negative breast cancer (TNBC) for whom immunotherapy is not an option. This new therapy significantly extended survival for some patients, representing a potential breakthrough in one of the most challenging forms of breast cancer.
- Hernexeos (zongertinib)
Sponsor- Boehringer Ingelheim
This therapy is the first orally administered targeted treatment for adults with previously treated HER2-mutant advanced non-squamous non-small cell lung cancer (NSCLC). HER2 (ERBB2) mutations, which occur in a subset of NSCLC patients, drive tumor growth by activating the tyrosine kinase domain, making the cancer more aggressive and often resistant to standard therapies.
The drug is a kinase inhibitor specifically approved for adults with unresectable or metastatic NSCLC whose tumors harbor these activating HER2 mutations, as confirmed by an FDA-approved diagnostic test, and who have already received prior systemic therapy. By directly targeting the HER2 pathway, this oral therapy offers a much-needed precision option, addressing an area of high unmet medical need and providing hope for patients with limited treatment alternatives.
Why Clinical Trial Participation Matters
Every FDA-approved cancer treatment begins with patients who take part in clinical trials. Their participation turns research into real therapies that improve cancer care for millions.
This World Cancer Day, it’s important to recognize that progress in oncology depends not only on innovation, but on people who step forward to participate. Explore current and upcoming breakthrough cancer clinical trials at TrialX.com or sign up for our volunteer registry to stay notified about the latest study opportunities.