🚀TrialX xPAND Supports Remote Data Collection for Clinical Research on Earth and in Space

TrialX xPAND is enabling decentralized clinical research here on earth and in space. In addition to helping clinical trial investigators collect study data remotely, we are proud to support pre and post flight data collection for historic space missions such as Axiom-2. EXPAND DB – the database built for TRISH using TrialX xPAND, is the first database of human health & environmental data from commercial space flights architected to support a variety of data types across a multitude of individual research studies. The database currently houses data from subjects across five space missions – Inspiration 4, MS-20, Axiom-1, Axiom-2 and Polaris Dawn. TrialX xPAND database equips space researchers to reuse and integrate research data across different research studies and unlock innovative actionable insights. Learn more.
What’s new at TrialX?
📈 DIGITAL HEALTH INNOVATION SUMMIT 2023

Paul Donnelly, our Chief Strategy Officer attended the Digital Health Innovation Summit in San Francisco, which connected thought leaders from Pharma, Healthcare, and the MedTech industry to discuss latest trends in clinical trial innovation. Presentations on digital therapeutics took centerstage with AI being at the forefront of many discussions. Clinical trials and health data are often subject to noisy endpoints with high misdiagnosis rates and limited ability to make predictions. Use of digital technologies in clinical trials (decentralized approach) has the potential to address these challenges and reduce operational complexity. Many companies showcased their digital solutions which are now proving to be able to detect health deterioration faster and optimize trial designs using data-driven insights. From designing digital inclusive protocols to creating efficiencies along the way, the theme revolved around improving health outcomes, faster drug development and personalized care. More and more companies including many from the pharma sector are making digital a priority realizing its capability in helping streamline clinical trials processes, provide better data, reduce in-clinic time spent, speed up recruitment and reduce prescreen failures and associated costs. Learn how TrialX is enabling decentralized clinical research here on earth and in space.
LATEST CURETALKS

Watch Dr. Carolyn Alexander, Associate Professor-OBGYN, UCLA, discuss the hopes and uncertainties around egg freezing – a procedure opted by an increasing number of women who are seeing it as an insurance to be able to start or expand a family more flexibly. Watch here.
🗃️PRODUCT UPDATES

Our latest iConnect release facilitates patient engagement and retention of the enrolled participants via emails. The system will now allow sites to send visit reminders and survey completion reminders to patients via emails, based on the enrollment date of each patient within the system and manage other study activities. Schedule a demo to learn more.
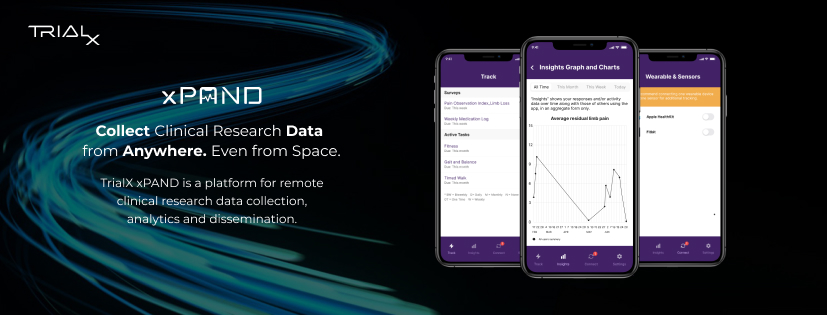
Using TrialX xPAND researchers at the Division of Plastic and Reconstructive Surgery, Northwestern University Feinberg School of Medicine developed Zing PainApp to monitor pain, medication use and quality of life domains such as mood, mobility, return to work, and sleep in patients with neurogenic pain and those with limb loss. The app successfully allowed the study team to gather eConsent, collect survey data and link to phone sensors and wearables to track steps data remotely for more than 2.5 years. Learn more.
👨💻 WEBINAR AVAILABLE ON DEMAND

In our recent webinar we shared how researchers are leveraging mobile health technology to gather eConsent, integrate wearable/sensor data into their study app and collect anonymous data from patients over time remotely.
🧬Featured Clinical Studies powered by TrialX iConnect
The REACTIVE Trial: The goal of this clinical trial is to determine the effectiveness of remote nonstress test (NST) compared to in-clinic NSTs in improving fetal testing completion rates. Participants will be randomized to either in-clinic NSTs or use of an FDA-approved remote monitoring belt for their pregnancy monitoring. Learn more.
Movement, Kinematics, and Social Information Processing: The purpose of this study is to examine whether differences in the way children make and perceive human body movements is associated with their social ability, and perhaps severity of autism spectrum disorder. Learn more.
RIDE+: This research study will help us see whether providing medical services in a mobile unit will help persons who inject drugs (PWID) start and stay on anti-HIV medicines and medicines for opioid use disorder better. Learn more.
🌎AROUND THE WEB
- 🍸Recycled pee and sweat on the ISS!
- 🚀Let there be dark. What will the first astronauts to mars eat?
- 🤖Coming soon! A wearable AI projector to replace smartphones & smartwatches. Cost?
- 🍜Swallowable gastric balloon to fill up the stomach, curb hunger.
- 🧬First gene therapy for Duchenne muscular dystrophy (DMD) approved. List price? 3.2 M!
🗣️TEAM UPDATES

Our Co-founder & CEO, Dr. Sharib Khan was invited to talk about TrialX’s success story and the impact of innovation on June 22, 2023 at the Orion Summit 2023. Listen to this exhilarating narrative of how TrialX came to be what it is today.
International Yoga Day 2023!

Embracing the power of mind, body and spirit, that’s our yoga enthusiast CEO, Dr. Sharib Khan turning clinical research upside down and attempting to balance work & living🧘♂️! A happy international yoga day!
We look forward to be able to bridge the gaps in clinical research here on earth and in space. Stay tuned for more updates as we continue to look up to the moon!