Adoption of Mobile in Clinical Trials – What do the experts say?
As proved now by several preliminary studies performed by prestigious organizations, use of mobile technology in clinical trials can improve efficiency of the clinical research industry. It offers several merits such as:
- Continuous acquisition of data, rather than at discrete intervals, giving more insight into a patient’s health.
- “Real-time” data gathering in “natural” surroundings providing knowledge of patient’s daily lives.
- Reduced clinic visits decrease patient’s and investigator’s burden.
- Decrease loss to follow up and increase patient recruitment.
Mobile technology is yet to be used up to its full potential in clinical trials. There are several barriers to the use of mobile devices in collecting clinical trial data – an important one being technological issues surrounding the devices used and the data gathered.
Clinical trial transformation initiative (CTTI) is a public-private partnership – co-founded by Duke University and FDA – that strives “To develop and drive adoption of practices that will increase the quality and efficiency of clinical trials”. Working towards this mission, in 2015, CTTI started a “Mobile Clinical Trials” (MCT) Project. One part of this MCT project, which is ongoing, is dedicated to collecting evidence on technological issues, from all stakeholders, based on their experience with mobile device use in clinical trials.
In this project, CTTI outlines the following data and device challenges surrounding the use of mobile in clinical trials:
Data Challenges:
- Origin/Source of data
- Integrity
- Transmission and audit trails
- Analysis and interpretation
- Security
- Data management – Backup and Archiving
- Submission – Making data available to FDA
Technological/Device Challenges:
- Device selection
- User authentication/access control
- Management of the physical device
- Calibration
- Validation
- Bring your own device (BYOD)
- Device failure
- Device reuse
- Performance considerations
- Monitoring outcomes
- Real-time safety signals
- Providing real-time data to study participants
CTTI which comprises more than 80 organizations from across the clinical trial enterprise with members from government agencies, industry representatives, patient advocacy groups, professional societies, investigator groups, and academic institutions – is collecting evidence based on expert meetings and interviews with sponsors and clinical trial investigators, as well as technical experts such as device manufacturers, data management experts, data security experts, and data analysts and biostatisticians.
Let’s have a look on the key lessons learned from these meetings with technical experts, investigators and sponsors so far.
Sponsors and Clinical Trial investigators say:
- Learn from small studies: There is a need to incorporate mobile devices into clinical trials early. Researchers should begin with small patient populations and collect low-risk data, to gain experience and knowledge prior to incorporation into a larger study.
- Understand data requirement at the onset: It is best to understand the type, frequency of collection, and relative quantity of data needed at the onset of the study.
- Reduce patient burden: There is a need to reduce intellectual as well as cost burden for the patient. This can be achieved by selecting patient-friendly interfaces and reducing the total number of devices needed per patient.
- Follow best practices: Researchers should follow best practices to protect patient identity with appropriate data security measures, just like for non-mobile clinical trials. The recommended best practices for studies using mobile devices for data capture do not differ significantly from many of the best practices that we already apply to clinical trials.
Technical experts say:
- Learn in-depth about the device: Practice due diligence with the vendor at the onset of device selection to ensure proper understanding of the device technology, data processing outputs, capacity of data storage, uploading frequency of the device etc. Understanding device performance and function was critical to ensure reliable, comparable, and accurate data across patient populations.
- Know if the device is fit for your purpose: Knowledge of the verification, validation, and mobile device calibration allows sponsors and investigators to ensure that the device is “fit for purpose”, and may allow earlier detection of irregular data.
According to CTTI, the experts involved also concurred that:
- Studies using mobile devices for data capture should not be held to higher standards than traditional trials.
- Mobile technology should not be used for data capture in a clinical trial just because it is available. It should be used in cases where it solves the purpose in a better way than the existing alternatives.
- This effort should strive to increase patient engagement in study design.
- The device should be able to collect data to support the endpoint for the particular patient population.
- Willingness by researchers to include mobile devices in appropriate clinical trials will lead to more evidence based learning and increased adoption.
Once additional insights are gathered, the project team will release recommendations which will help overcome challenges that are currently hindering the wider adoption of mobile technologies in clinical trials. We eagerly await these recommendations to be released in early 2018!
Jennifer Goldsack from CTTI will present the findings of its other MCT project focussing on novel endpoints at #dpharm2017 this year.
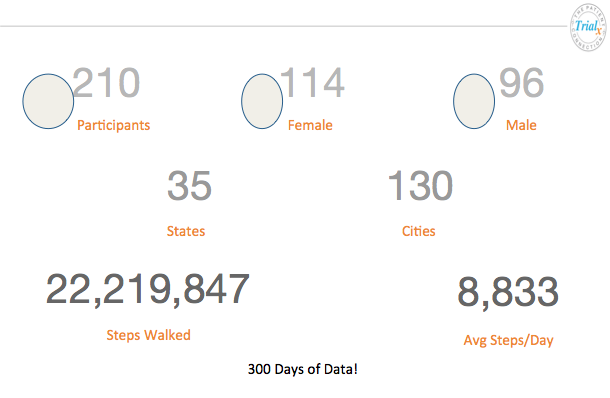
Last year in Feb 2016, we conducted our very own study – America Walks – an observational study to determine walking behavior of individuals in the United States. The study successfully collected walk data from about 210 participants using a, first of its kind, cross-platform app that worked both on android and iOS phones. The preliminary results of this study was presented at the Mobile in Clinical Trials conference last year in 2016, covered in detail by Mobihealthnews. You can learn about the preliminary results of America Walks Study here.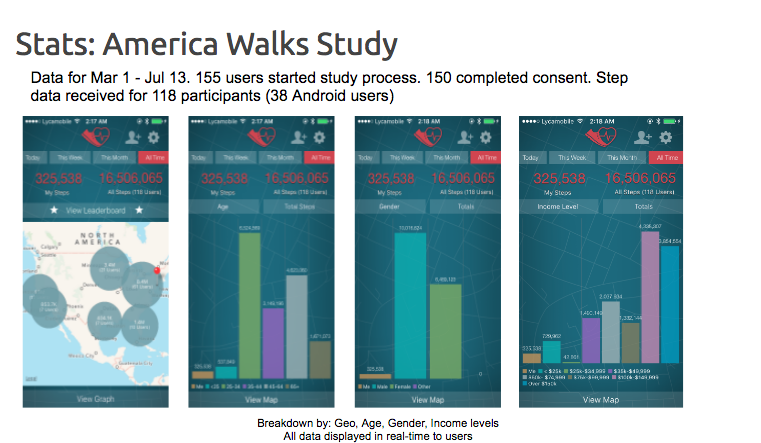
This year, our CEO, Dr. Sharib Khan will present Appbakery, a DIY research study app building platform at @conferenceforum at #mobileclin2017 in Boston. The Appbakery platform allows researchers to design, create and deploy their entire mobile study app for their research studies, using a web interface, without needing to write any code.
If you are a researcher or physician looking to start a mobile based research study, and collect survey or sensor/wearables data using smartphone apps, you might want to learn more about Appbakery. Get in touch with us!