Unveiling Innovation: TrialX’s Highlights from SCOPE Summit 2024

We participated in the 15th annual Summit for Clinical Operations Executives (SCOPE) recently. With over 3000+ attendees from 800+ companies, it was a huge success! Representing TrialX as a leading innovator in healthcare technology, our CSO, Paul Donnelly, engaged with industry leaders, exchanged insights, and shared about our solutions aimed at revolutionizing the clinical trial landscape.
Our participation was marked by the unveiling of our latest innovation – an AI-powered solution designed to simplify clinical trial information for patients (find the AI magic at the bottom right!). Navigating clinical trials can be daunting for many. Our AI-driven approach aims to dismantle these barriers and foster greater participation. This game-changing tool exemplifies our commitment to driving patient-centric innovation and advancing the future of clinical research.
Through our LinkedIn post, we invited stakeholders to get a sneak peek into our AI powered innovation and be a part of the innovation journey. We look forward to feedback and insights which will help us refine this transformative tool.
Key Takeaways from #SCOPE2024
SCOPE Summit 2024 proved to be a melting pot of thought-provoking discussions, cutting-edge presentations, and invaluable networking opportunities. The sessions kickstarted with a multi-stakeholder panel on integrating research into the care continuum where a diverse set of leaders representing health authorities, sponsors, sites, and industry consortia discussed opportunities and barriers to giving patients access to clinical research as part of the care continuum. Also, the idea of looking at other industries for best practices and challenging the status quo was a topic of conversation.
Here’s a glimpse into other highlights & key takeaways from the event:
Patient-Centricity in Clinical Research
One of the overarching themes at SCOPE Summit 2024 was the growing importance of patient-centric approaches in clinical research. Sessions delved into strategies for enhancing patient engagement, improving recruitment and retention, and prioritizing patient needs throughout the trial journey.
Pfizer’s Rob Goodwin talked about his organization’s aspirations to reduce their operational cycle times, and having to move quicker without compromising quality. He also touched on partnering with emerging solutions to centralize a patient centric experience, optimize content and channels, and maximizing site volumes & capacity. DCT’s and the PfizerLink Registry was also well received.
Innovations in Digital Health and Remote Monitoring
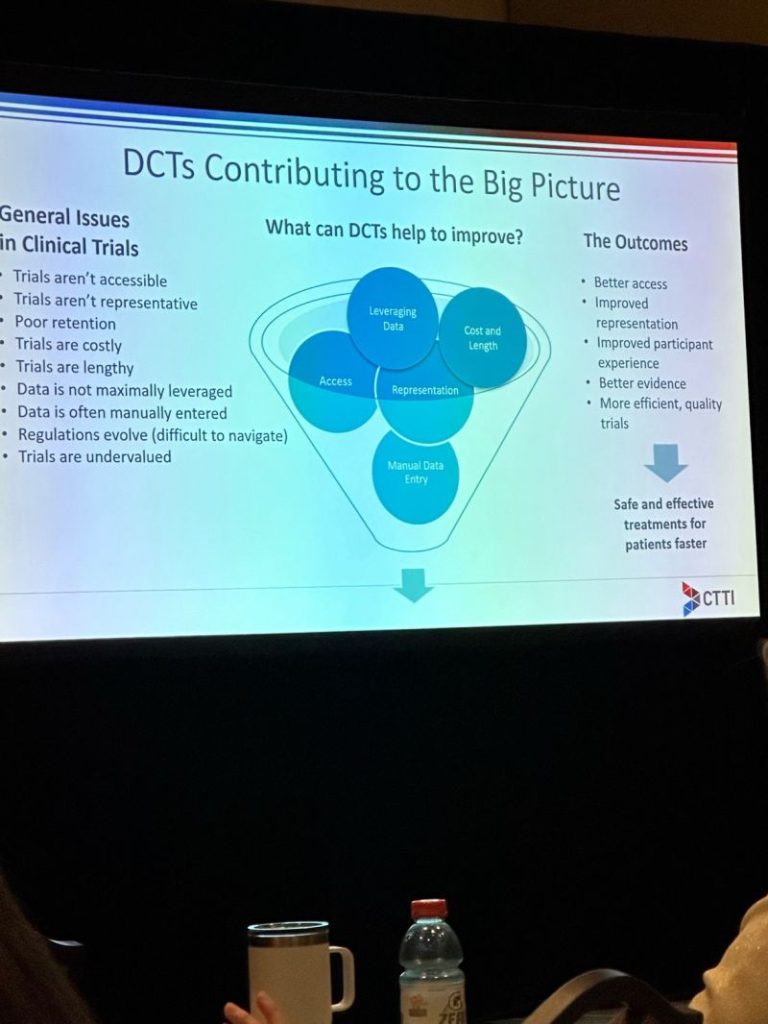
With the rise of digital health technologies, there was a considerable bustle around innovations in remote monitoring, wearables, and telemedicine. Experts explored the potential of these technologies to streamline data collection, enhance patient monitoring, and drive efficiencies in clinical trials. For example: The Clinical Trials Transformation Initiative (CTTI) talked about its mission to drive best practices to increase quality of clinical trials – making clinical trials patient centered, easily accessible, fully integrated into health processes, designed with quality, leveraging all the data, and improving population health. The discussions highlighted that technologies like decentralized clinical trials (DCTs) are designed to create efficiencies, not to be a burden.
We shared how TrialX is transforming remote data collection with its decentralized clinical trial solutions that are powering clinical studies here on earth and in space!
AI and Machine Learning in Clinical Trials
The intersection of artificial intelligence (AI) and clinical trials emerged as a focal point of discussion. Presentations and panel discussions delved into the transformative potential of AI and machine learning in optimizing trial design, accelerating patient recruitment, and unlocking actionable insights from data. Brian Martin, Head of AI, R&D Information Research, Research Fellow, AbbVie, Inc. moderated an insightful panel discussion brainstorming where and when we should use generative AI in Clinical Trials.
AI is a hybrid environment, crossing the human/computer interface. Tools are now available to inform protocol design, but still need human intervention when making final decisions. The context and interpretation is key. Now we can do things faster and more efficiently while focusing on the more strategic elements of running trials. It’s no longer focused on whether we can build a model to predict, it’s – is it the right model?

Regulatory Landscape and Compliance Challenges
Regulatory compliance and evolving guidelines were also burning topics at SCOPE Summit 2024. Sessions provided updates on regulatory developments, compliance best practices, and strategies for navigating the increasingly complex regulatory landscape in clinical research. In a fireside chat with FDA on modernizing clinical trials, Ken Getz, Executive Director and Professor, Tufts Center for the Study of Drug Development, Tufts University School of Medicine, shared that many of the new products that FDA is being asked to evaluate aren’t easily evaluated using traditional approaches. Therefore, the FDA has been engaged in a comprehensive effort to enable the modernization of clinical trials.
Diversity and Inclusion in Clinical Trials
There was a growing recognition of the need for greater diversity and inclusion in clinical trials. Discussions centered around strategies for improving diversity in trial participant populations, addressing disparities in access to clinical research, and ensuring equitable representation across all demographic groups. We shared how TrialX’s patient recruitment management solution is facilitating diversity and inclusion by allowing more granular patient outreach and enabling sponsors and sites to create an end-to-end multilingual experience for patients. Also, the use of registries was top of mind for many, because having more data on diverse audiences helps to better identify the right patients at the right time, and TrialX has been successfully managing this for many client initiatives.
As the SCOPE Summit 2024 drew to a close, we left inspired by the insightful discussions, energized by new connections, and emboldened in our mission to transform the clinical trial landscape through innovation and collaboration. With momentum from SCOPE Summit 2024 we look forward to continuing our journey of driving positive change in healthcare.
For more information on TrialX’s innovative solutions, visit trialx.com


