Understanding Agentic AI: How It’s Changing the Landscape of Clinical Trials

In 2022, OpenAI launched its first large-scale AI tool—an innovation that quickly reshaped how industries approached automation, creativity, and problem-solving. Since then, artificial intelligence has become part of everyday workflows, turning time-intensive tasks into faster, data-driven processes. That transformation has entered a new phase with the rise of agentic AI. Unlike traditional systems that act only when prompted, agentic AI operates with a degree of autonomy—setting goals, reasoning through tasks, and taking action to complete them. It marks a shift from simple automation toward more adaptive, intelligent systems capable of meaningful collaboration with people.
As one industry observer noted, “The shift to the agentic enterprise is more than a technology story—it’s about empowering teams to take intelligent action that truly impacts patients.” In clinical research, where nearly 86% of clinical trials fail to meet enrollment targets on time, this shift could be transformative.
From Clinical AI to Agentic Intelligence in Clinical Trials
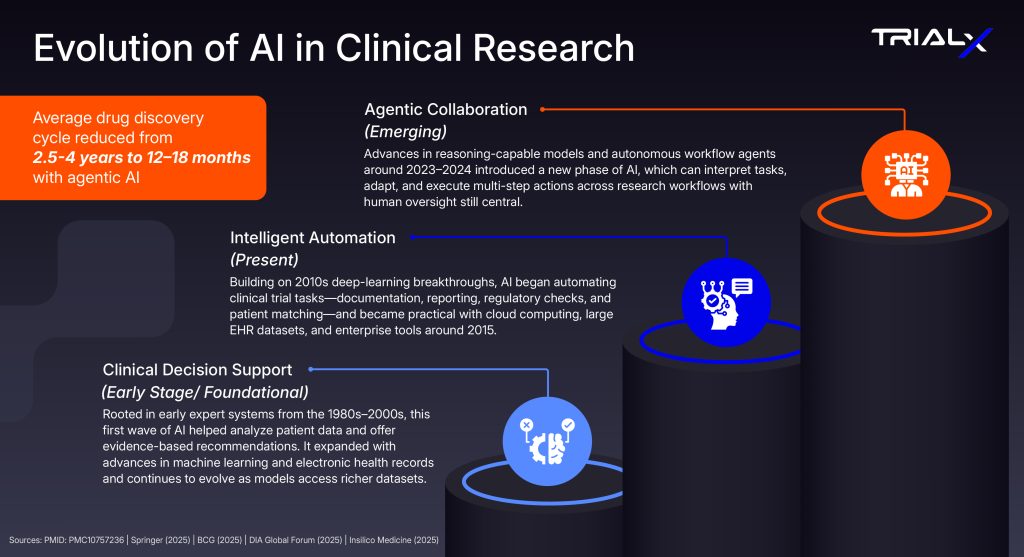
Early artificial intelligence systems in clinical research—such as Clinical Decision Support Systems (CDSS)—paved the way for today’s intelligent automation. These tools analyzed patient data to guide evidence-based trial and treatment decisions, offering researchers faster insights and reducing manual interpretation.
Example: IBM Watson for Oncology evaluated cases against 15 million pages of literature and trial data to suggest personalized therapy options, while Epic’s Electronic Health Record ((EHR) System, helped researchers flag dosing issues, detect protocol deviations, and track adverse events. Though largely rule-based, these systems marked the first steps toward autonomy—interpreting complex data and acting within human oversight.
Building on that foundation, agentic AI now extends these capabilities across the entire trial lifecycle. It can automate documentation, accelerate protocol drafting, and enhance drug discovery. Companies like Insilico Medicine, Atomwise, and BenevolentAI have shown that AI can reason through hypotheses, design molecules, and test compounds autonomously—compressing early development timelines from 2.5–4 years to just 12–18 months. This upstream acceleration directly benefits clinical trials, enabling faster protocol setup, patient stratification, and adaptive monitoring once trials begin.
Together, these systems form a continuous learning loop:
Data → Reasoning → Decision → Documentation → Feedback → Adjustment
Experts anticipate that in the next five to ten years, such AI will be a core part of trial operations, supporting investigators as intelligent, compliant, and transparent collaborators that accelerate discovery and improve outcomes.
So, What Is Agentic AI?
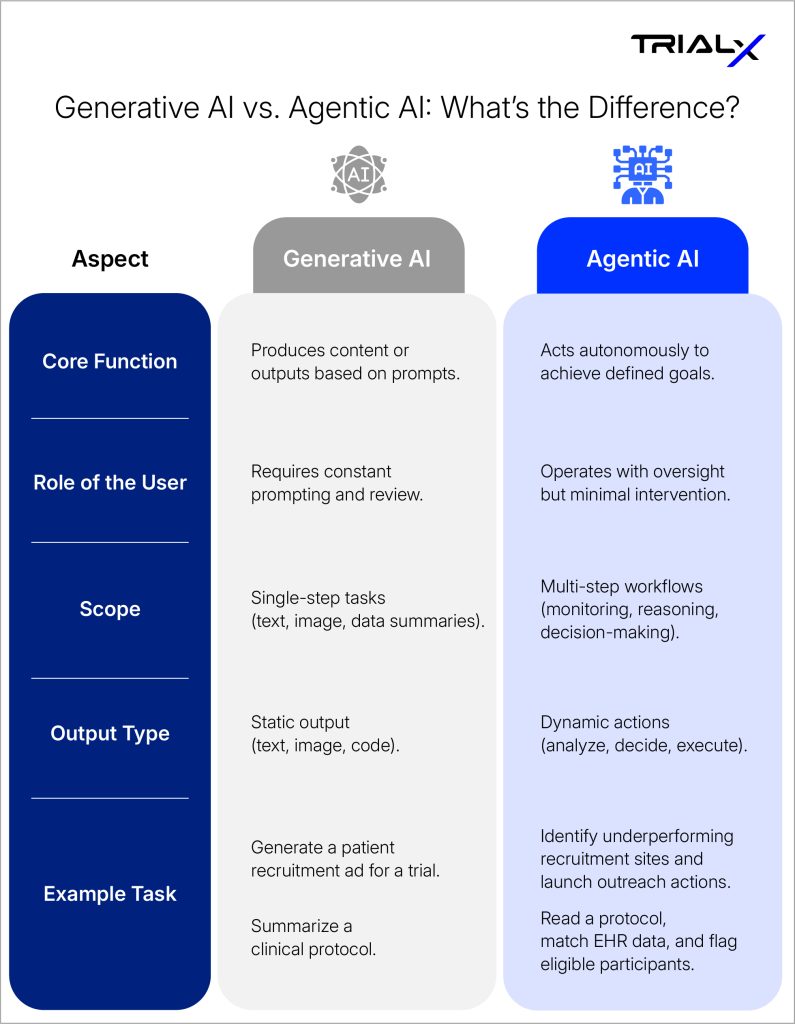
Agentic AI refers to AI systems that operate as independent “agents”—software entities capable of setting goals, reasoning about progress, and acting autonomously to complete multi-step tasks.
Think of it as the difference between asking a digital assistant for a single answer versus giving it a mission. A traditional AI might summarize a clinical protocol; an agentic AI can read the protocol, extract eligibility rules, compare them with EHR data, and flag potential participants—all without human prompting.
In other words, agentic AI doesn’t just wait for input—it takes initiative.
Generative AI vs. Agentic AI: What’s the Difference?
Agentic AI in Patient Recruitment in Clinical Trials
Patient recruitment remains one of the most persistent challenges in clinical research, often resulting in delays, higher costs, and slower progress in bringing new therapies to patients. Agentic AI offers a way to simplify and accelerate this process by coordinating multiple recruitment activities within a single, connected workflow.
How Agentic AI Supports Patient Recruitment
- Creates an integrated workflow by linking protocol review, patient data analysis, and outreach management into one coordinated process—reducing manual work and speeding up recruitment timelines.
- Improves patient matching by comparing detailed eligibility criteria with electronic health records and ranking potential participants based on multiple clinical factors.
- Personalizes outreach dynamically, adjusting communication tone, timing, and content based on engagement data and demographics to enhance diversity and retention.
- Monitors progress automatically, detecting delays across sites in real time and redirecting recruitment efforts where needed to maintain steady enrollment.
Recent studies and pilot programs also highlight the benefits of agentic AI in patient recruitment:
- Recruitment pipeline efficiency improved by 46% across global firms adopting agentic AI (2025 study).
- Companies including Pfizer, Roche, and AstraZeneca now use agentic AI in 38% of their ongoing clinical trials to automate patient follow-ups, log adverse events, and ensure compliance.
- McKinsey notes agentic AI can boost clinical development productivity by 35-45%, implying meaningful cost and time efficiencies across all clinical trial functions.
How Regulators Are Adapting to Agentic AI
As industry adoption grows, another important indicator of progress is how regulators are beginning to incorporate AI into their own internal processes. Recently, the FDA expanded its internal AI program, introducing agentic AI tools to support reviewers, investigators, and scientific staff.
What’s notable is how the agency frames these systems: not as autonomous decision-makers, but as structured, goal-oriented assistants that help streamline complex work. These AI tools are currently being used to support tasks such as:
- Coordinating meetings
- Validating review
- Assisting with pre-market evaluations
- Monitoring post-market safety signals
- Preparing documentation for inspections and compliance activities
The FDA has emphasized that these systems:
- Are optional and used voluntarily
- Operate within a secure GovCloud environment
- Do not train on regulatory submissions or confidential sponsor data
- Remain under strict human oversight at all times
To encourage safe exploration of new applications, the agency also launched an internal “Agentic AI Challenge,” inviting staff to prototype agents that address real workflow bottlenecks.
The Human-in-the-Loop: Supporting, Not Replacing
Across the field, experts agree that agentic AI delivers real value only when paired with thoughtful human oversight. Pfizer’s experience reinforces this: their teams use AI to handle large volumes of trial and patient data, streamlining analysis, improving collaboration, and getting key insights to decision-makers faster.
At the 2025 Patients as Partners (PASP) conference, much of the discussion focused on keeping AI technology powerful yet people-centered.
Sharib Khan, CEO and Co-founder, TrialX, summed it up during The View panel:
“Our big picture says: generated by AI, reviewed by humans. That’s the fundamental paradigm shift.”
He explained how TrialX’s AI tools can now turn a clinical trial protocol into a complete, multilingual study website within hours instead of weeks. Every output still goes through expert review to ensure accuracy, clarity, and patient relevance. This approach combines the efficiency of AI with the judgment and empathy of human oversight.
Overall, the direction is clear: agentic AI is designed to support, not replace, people. By reducing administrative work and improving access to insights, it helps teams concentrate on study design and patient experience.