Human Health in Space Missions, Decentralized Clinical Trials Webinar, Welcoming New Members and More…

This year’s SCOPE was amazing and well attended with over 3100 people coming from around the globe representing sponsors, CROs, suppliers, consultants and most importantly patients. Coming off last year where there was a fraction of attendance compared to this year, many including TrialX, found it successful on many fronts. Here’s what we learnt at SCOPE 2023.
👨💻UPCOMING WEBINAR

Virtual platforms, generally downloadable to the patients’ smartphones, make it possible for patients to report from their homes. By deploying mobile research study apps, DCTs (Decentralized clinical trials) have the scope to collect more data from patients over time than in a traditional site-based clinical study, providing data which is more akin to a real-world setting. The apps can be used for data collection, include automated checks to reduce errors and have notification capabilities, which help maintain patient engagement increasing compliance. Join us in this webinar where you will learn:
- How to leverage eConsent for your studies?
- How to integrate wearable/sensor data into your study app?
- How researchers from New York University, Northwestern University and Albert Einstein School of Medicine are leveraging mobile research study apps to collect patient generated data and what this could mean for your study?
WHAT’S NEW AT TRIALX?
LATEST CURETALKS

In this new episode of TrialX CureTalks watch Dr. Mathias Basner of University of Pennsylvania share interesting facts and study observations about how human health and behavior changes in the microgravity environment of space. Watch here.
🎍WELCOMING NEW MEMBERS
Charles Speno joins TrialX as a Senior Director of Sales and Marketing to help accelerate our vision of connecting patients to clinical trials, facilitate clinical research through remote data collection and raise research awareness. Charles brings in 20 years of experience and a demonstrated history in business development in the pharmaceutical industry. Starting his career as a special education teacher, Charlie has largely dedicated his time towards developing clinical trials patient enrollment and retention services. At TrialX he aims to utilize technology to optimize clinical research operations and compress the time from concept to care option.
Lisa Allison joins TrialX iConnect team as iConnect Implementation Manager. Lisa is a passionate clinical operations leader with more than 20 years of experience leading mission driven projects. Her experience includes working with clients across the US to adapt projects and products for end-users in research, quality accreditation, and process improvement. With a strong background in oncology care as a RN, she is passionate about ensuring equity in access to care and clinical research. She also works locally with an in-home elder-care organization in Colorado Springs.
Featured Clinical Studies powered by iCONNECT
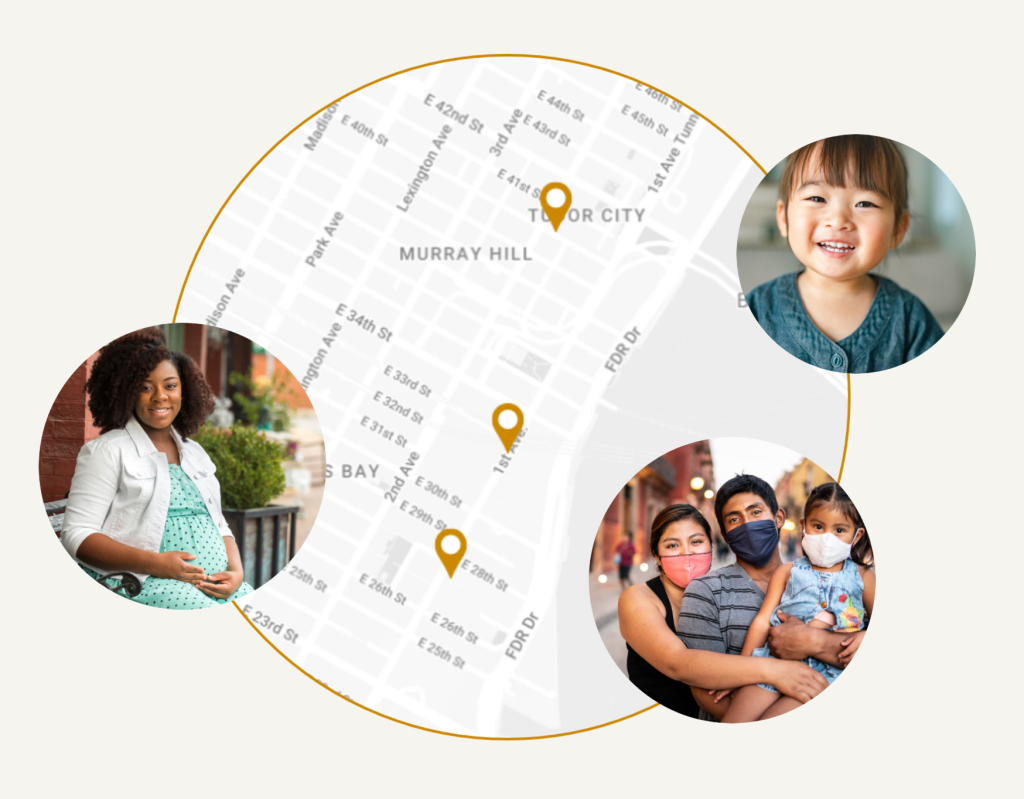
The RECOVER Study: The National Institutes of Health (NIH) created RECOVER (Researching COVID to Enhance Recovery) Initiative to learn about the long-term effects of COVID. The initiative aims to recruit up to 40,000 participants and thousands of children and adults including pregnant people have joined various RECOVER studies. Learn more
The Your View Study: Researchers at Purdue University want to learn more about what children see when playing and interacting with their parents. Knowing more about how children view the world may help us better understand early influences on learning. Learn more
Digital Data and Heart Health Study: This research study is exploring about how digital data (i.e. social media, online searches, and step tracking data) are associated with Coronary Heart Disease (CHD) and related risk factors in order to develop a more precise CHD risk predictor tool. Learn more
As we begin 2023, we aim to take clinical trials patient recruitment and remote data collection to the next level towards our mission of connecting over a million patients to clinical research.
Stay tuned for more updates!
Team TrialX