TrialX CEO Turns Clinical Research Upside Down with Appbakery at #Dpharm2017 Day1 – #MobileClin2017
Our CEO, Dr. Sharib Khan did the unimagined and gave true meaning to DISRUPTION when he did a headstand while presenting Appbakery – turning clinical research upside down literally – at the Mobile in Clinical Trials conference in Boston (#mobileclin2017)
Day 1 of the Disruptive Innovations conference this year #dpharm2017 was dedicated to talks around use of mobile in #clinicaltrials.
Our demo ended with a big applause from the audience. Here’s the link to the video.
In the live tech demo round, Dr. Khan asked one of the attendees to try out Appbakery – our DIY research study app building platform – to build an app in 5 minutes. The Appbakery platform allows researchers to design, create and deploy their entire mobile study app for their research studies, using a web interface, without needing to write any code.
Photo Courtesy: Conference Forum
Photo Courtesy: Conference Forum
The day at the conference in Boston started with opening remarks by Daniel Karlin, MD Head of Experimental Medicine, Informatics, & Regulatory Strategy, Pfizer Inc., followed by an informative session on how to overcome cultural barriers in mobile implementation. This session was moderated by Jeff Lee, CEO of mProve Health. Jane Myles of Roche/Genentech can be seen interacting with Munther Baara from Pfizer, Jacob LaPorte from Novartis, and Alex Simmonds from Bristol-Myers Squibb.
In the discussion Munther Baara suggested that we should work bottom-up & top-down & go case by case with use of digital technology in clinical research. He reminded that cost is an important consideration in and no one size fits all in this field. He also said, “Don’t shy away from implementing eConsent outside the United States” and mentioned that “It’s possible to take a hybrid approach to eConsent”.
In another session @Medidata‘s Anthony Costello examined eConsent lessons, challenges & predictions.
Courtesy: Conference Forum
Courtesy: Conference Forum
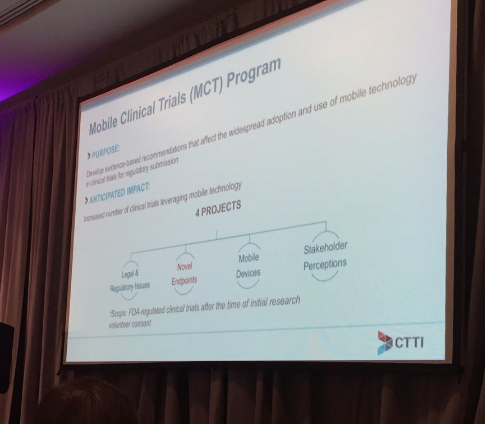
Jennifer goldsack of CTTI, outlined CTTI’s MCT program and talked about the pathway to develop novel endpoints from data generated by use of mobile in clinical trials.
Courtesy: Conference Forum
Courtesy: Conference Forum
Courtesy: Conference Forum
Many experts from prestigious educational institutions and companies in the field spoke about their experiences with use of mobile in clinical trials and shared their technologies contributing to increased patient engagement. Experiences and challenges with the famous eHeart study by UCSF, GSK’s PARADE study, and Roche’s Parkinson’s study were shared.
Following the hashtag #mobileclin2017 on twitter outlines how the day went @confereneceforum in Boston. You can also find the the event recap here.
Some of the major concerns around mobile apps in research talked about at the conference were:
- Data integrity
- Bringing data back to sponsor the company
- Handling data coming in in various languages
- Android variability
Today on September 7 at 3:40 PM, Dr. Sharib Khan will talk about ‘Transforming Patient Recruitment at the Enterprise Level with iConnect 2.0’ – our clinical trial management system currently being used by NYU, UPENN etc.
We look forward to more fruitful interaction and networking at #dpharm2017 in the next couple of days.
If you are a researcher or physician looking to start a mobile based research study, and collect survey or sensor/wearables data using smartphone apps, you might want to learn more about Appbakery. Get in touch with us!