Transforming Remote Data Collection in Clinical Research – SAYSTOP Study App for Investigating Antibiotics Resistance

Over the past decade, clinical research has undergone a digital revolution, with decentralized clinical trials (DCTs) and remote data collection reshaping how studies are conducted.
Traditionally, research relied heavily on site-based visits, paper documentation, and retrospective data collection, often leading to inefficiencies, delayed insights, and increased patient burden. Now, with the rise of mobile health apps, wearable devices, and real-time data capture technologies, researchers can access patient-reported data instantly, improving study accuracy and compliance.
TrialX has been at the forefront of this shift, developing advanced digital tools to streamline study execution, improve patient engagement, and enhance data collection. Clinical research study app such as – The Study of Antibiotics You Stop for Treatment of Pyelonephritis (SAYSTOP) App – created for a leading research study led by the University of Iowa and UCLA’s Olive View Medical Center, is a testament to how technology can empower both researchers and patients in addressing major healthcare challenges—such as antibiotic resistance.
Traditional Approach to Antibiotic Usage and Research Challenges
For decades, medical guidelines have advised patients to complete their full course of antibiotics, even if they feel better before finishing. This approach aims to ensure that all bacteria are eliminated and prevent recurrence. However, stopping antibiotics early when feeling better—can contribute to antibiotic resistance, a global health crisis responsible for millions of deaths each year.
In cases like acute uncomplicated pyelonephritis (a type of urinary tract infection, or UTI), many patients recover before finishing the full prescribed course of antibiotics. But is it safe to stop early? The SAYSTOP study seeks to answer this question by comparing outcomes between patients who discontinue antibiotics once symptoms resolve and those who complete the full course.
Running such a study, however, comes with challenges. Traditional clinical trials require patients to travel to research sites for screenings, check-ups, and data collection. While this ensures controlled conditions, it also limits participation, increases dropout rates, and slows data collection—making trials more expensive and less efficient.
What’s Changing? The Role of Digital Solutions and Decentralized Trials
To address these barriers, researchers are turning to DCTs, which use digital tools like mobile apps and wearables to track patient progress remotely. These tools can now collect data through mobile health applications and remote patient monitoring tools, eliminating the need for in-person follow-ups and paper-based reporting.
According to a McKinsey analysis, the adoption of decentralized approaches has surged in recent years, with 48 to 95 percent of sponsors incorporating at least one decentralization element in Phase III trials,.
Regulatory bodies like the FDA have acknowledged the transformative potential of digital health and have introduced guidelines supporting electronic data capture (EDC), telemonitoring, and decentralized study models. This shift allows clinical trials to be more inclusive, patient-friendly, and scalable, particularly for studies like SAYSTOP, which require longitudinal symptom tracking.
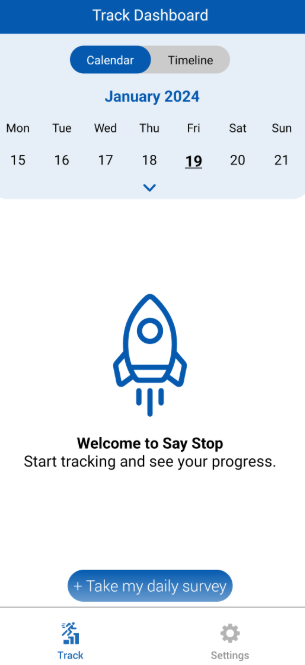
About the SAYSTOP Study App

The SAYSTOP Study app will help patients participate in this clinical trial from the comfort of their homes, allowing real-time symptom monitoring, medication tracking, and data collection without frequent clinic visits. During the study, participants will receive antibiotics packaged in two separate blister packs. They will follow a structured treatment plan, switching medications during the study period while tracking their symptoms and adherence through the app. The app ensures seamless participation by allowing patients to:
- Log Symptoms Daily – Participants record how they feel each day, helping researchers track the progression of symptoms and assess antibiotic effectiveness.
- Track Medication Use – The app enables accurate logging of antibiotic intake, ensuring adherence monitoring and better compliance.
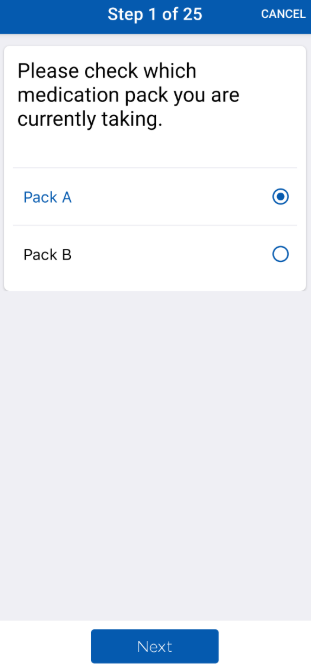
- Receive Automated Reminders & Alerts – The app sends a daily reminder prompting participants to complete their symptom survey. If the survey remains incomplete by noon, a second notification is triggered to ensure timely data entry and improve study compliance.
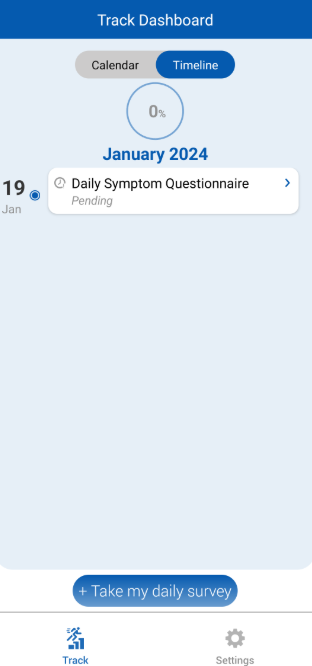
- Enable Seamless Remote Monitoring for Researchers – Investigators can remotely access patient-reported outcomes in real time, reducing the need for in-person visits while maintaining high data quality.
The Power of Decentralized Clinical Trials
Decentralized trials have transformed how clinical data is collected, making research more patient-friendly and efficient. Remote data collection plays a key role in this transformation, offering several advantages:
Continuous & Real-Time Data Collection
Traditional clinical trials rely on scheduled site visits for data collection, which can miss important changes in a patient’s condition. Remote data collection, often powered by digital health technologies (DHTs) like wearables and mobile apps, enables real-time monitoring of patient health. This leads to richer datasets, capturing daily fluctuations and long-term trends that improve the accuracy of trial outcomes.
Long-Term Insights for Better Decision-Making
Remote data collection makes it easier to gather long-term evidence on treatment effectiveness. This is especially valuable for health technology assessments (HTAs) and regulatory bodies that require ongoing data to support drug approvals and reimbursement decisions. By allowing continuous patient monitoring beyond the trial period, decentralized trials can provide stronger evidence for the real-world benefits of new treatments.
Greater Diversity & Accessibility
Decentralized trials reduce geographical and logistical barriers to participation. Patients from rural areas, those with mobility challenges, or individuals with demanding schedules can contribute to research without traveling to a clinical site. This broader accessibility helps recruit a more diverse participant pool, improving the generalizability of trial findings across different populations.
Improved Patient Convenience & Engagement
By minimizing the need for frequent in-person visits, remote data collection reduces the burden on participants. Patients can provide data from home using digital tools, making the trial experience more convenient. This increased ease of participation often leads to better engagement, higher retention rates, and more complete datasets, ultimately strengthening the trial’s reliability.
Stronger Regulatory & Industry Support
Regulatory agencies and industry stakeholders increasingly recognize the benefits of remote data collection in clinical research. The ability to collect high-quality, real-world data aligns with evolving regulatory expectations, making decentralized trials an attractive option for sponsors looking to streamline trial approval processes and generate meaningful evidence efficiently.
Transforming Remote Data Collection On Earth And Beyond
At TrialX, we are committed to advancing decentralized clinical trials by breaking down participation barriers and enhancing the research process. Our expertise in remote data collection has supported a wide range of studies at other leading organizations such as Northwestern University and Columbia University, including collaborations that extend into unique environments such as space.
Our remote data collection platform has been instrumental in several groundbreaking space missions. In collaboration with the Translational Research Institute for Space Health (TRISH), we developed EXPAND database, an open, standards-based data repository designed to collect and manage health data from commercial spaceflight missions. Since September 2021, EXPAND database has housed over 1 million data points from 15 civilian astronauts across six commercial missions and one analogue mission. These missions include Inspiration-4, MS-20, Axiom-1, Axiom-2, Axiom -3, and Polaris Dawn.
Click here to learn more about our remote research data collection platform, request a demo, and build a research study app with us.