2025: A Breakthrough Year for Drug Innovation – 10 First-in-Class and First-Ever Treatments Approved in 2025
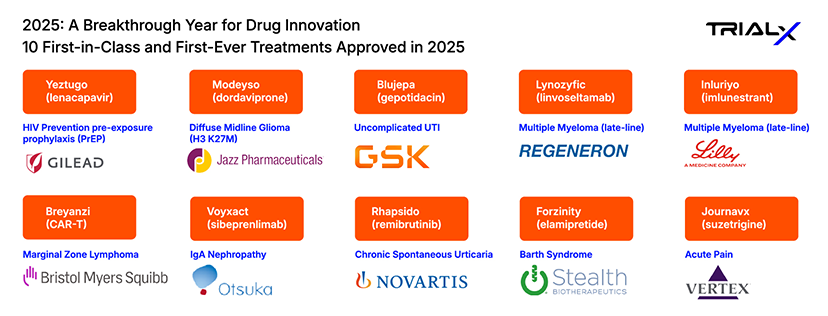

2025 has seen 43 novel FDA drug approvals – new drugs never before approved or marketed in the U.S for a condition – introducing high-impact therapies that are redefining what “breakthrough” truly means. This year’s portfolio highlights durable formulations (less frequent dosing), precision treatments guided by genetics or biomarkers, non‑opioid options, rare disease therapies, and medicines with broad public-health impact.
In this blog, we highlight the top 10 first-in-class and first-ever therapies approved by the FDA in 2025. These breakthrough therapies introduce new ways to treat conditions that have long had limited options.
- Yeztugo (lenacapavir)
Sponsor: Gilead Sciences
Yeztugo represents a major advance in HIV prevention as the first FDA-approved twice-yearly injectable option for pre-exposure prophylaxis (PrEP) in the United States. In the Phase 3 PURPOSE-1 and PURPOSE-2 trials, lenacapavir demonstrated very high efficacy, with the vast majority of participants remaining HIV-negative across diverse global study populations. By combining long-acting dosing with a novel capsid-inhibitor mechanism, Yeztugo expands the range of biomedical prevention strategies available today. Reflecting its scientific and public-health significance, lenacapavir was named Science magazine’s “Breakthrough of the Year” in 2024.
- Breyanzi (lisocabtagene maraleucel)
Sponsor: Bristol-Myers Squibb
Breyanzi is a CAR-T cell therapy, which takes a patient’s own immune cells and reprograms them to recognize and attack cancer cells — essentially turning the patient’s immune system into a precision cancer-fighting force. On December 4, 2025, the FDA approved Breyanzi as the first and only CAR-T therapy for adults with relapsed or refractory MZL, a rare type of slow-growing non-Hodgkin lymphoma that can be difficult to treat after multiple relapses. In the key clinical study (the TRANSCEND FL-MZL cohort), 95.5% of patients who received a single infusion of Breyanzi after preparatory chemotherapy showed very high response rates, demonstrating a consistent safety profile, while many experienced deep and lasting remissions. This one-time treatment option offers hope for people whose cancer has returned or stopped responding to standard therapies — a population with limited alternatives.
- Modeyso (dordaviprone)
Sponsor: Jazz Pharmaceuticals
Modeyso is the first FDA-approved systemic therapy specifically for diffuse midline glioma with the H3 K27M mutation, filling a critical gap in care for a cancer that mainly affects children and young adults and often carries a poor prognosis. Approved under the FDA’s accelerated approval pathway in August 2025, Modeyso is taken as a once-weekly oral medicine that can reach patients outside of hospital settings. In clinical studies, about 22% experienced partial or minor shrinkage of their tumors; of these patients, 73% had shrinkage of their tumor that lasted more than six months, offering hope where options were once extremely limited.
- Voyxact (sibeprenlimab)
Sponsor: Otsuka Pharmaceutical
Voyxact targets the APRIL(A PRoliferation-Inducing Ligand) protein, a key driver of pathogenic IgA production in IgA nephropathy. In the Phase 3 VISIONARY trial, monthly treatment led to approximately a 51% reduction in proteinuria at nine months compared with placebo. Proteinuria reduction is associated with slowing kidney function decline, making this a clinically meaningful outcome. The therapy is administered once every four weeks via subcutaneous injection, which can be done at home. Voyxact represents a new mechanism of action beyond supportive care, addressing an underlying immune driver of IgAN.
Sibeprenlimab is being studied in a Phase 2 trial for IgA nephropathy—find out more here.
- Blujepa (gepotidacin)
Sponsor: GSK (GlaxoSmithKline)
Blujepa is the first new oral antibiotic class approved for uncomplicated UTIs in nearly 30 years, marking a rare and important advance in infectious disease treatment. Most UTI antibiotics in use today belong to older drug classes, many of which are increasingly affected by antibiotic resistance.
In the EAGLE-2 and EAGLE-3 clinical trials, Blujepa demonstrated clinical effectiveness comparable to commonly used treatments, while offering a much-needed new option in an area where innovation has been limited for decades. Given how frequently UTIs occur and how often resistance complicates treatment, introducing a new antibiotic class has meaningful implications for everyday patient care.
- Rhapsido (remibrutinib)
Sponsor: Novartis
Rhapsido is the first oral targeted therapy approved for Chronic Spontaneous Urticaria (CSU) that works through Bruton’s tyrosine kinase (BTK) inhibition. Until now, patients whose symptoms did not respond to antihistamines often had to rely on injectable biologics or long-term steroids, which can be inconvenient or come with safety concerns. Rhapsido offers a once-daily oral alternative, giving patients a simpler, non-injectable option. By blocking BTK, Rhapsido interferes with immune signaling pathways involved in mast cell and basophil activation — key drivers of hives and itching in CSU. In clinical trials, patients treated with remibrutinib experienced meaningful reductions in itch severity and hive count, including those who had failed standard treatments. This makes Rhapsido especially important for people with difficult-to-control diseases.
- Lynozyfic (linvoseltamab)
Sponsor: Regeneron Pharmaceuticals
Lynozyfic represents a new generation of precision immunotherapy for multiple myeloma. It is a bispecific antibody, designed to bind both cancer cells and T cells at the same time, physically bringing the immune system into close contact with the tumor. This “immune-redirecting” approach helps the body’s own defenses recognize and kill myeloma cells more effectively. For patients whose disease has returned or stopped responding after multiple treatments, options are often exhausted. In clinical studies, linvoseltamab showed strong anti-tumor activity in heavily pretreated patients, including deep and durable responses in some cases.
- Forzinity (elamipretide)
Sponsor: Stealth Biotherapeutics
Forzinity is the first FDA-approved therapy for Barth syndrome, making it a landmark in rare disease treatment. Elamipretide works by targeting mitochondrial function, helping cells produce energy more efficiently, which is central to the symptoms of Barth syndrome. Clinical studies show elamipretide can improve mitochondrial function and boost muscle strength by 45% and heart function by 40% in patients with Barth syndrome, addressing critical aspects of this rare disease. The approval represents a significant milestone for patients and families who had no disease-specific therapies available.
- Inluriyo (imlunestrant)
Sponsor: Eli Lilly and Company
Inluriyo is FDA-approved as an oral selective estrogen receptor degrader (SERD) for adults with ESR1-mutated, ER+, HER2– advanced or metastatic breast cancer. ESR1 mutations occur in an estimated 50% of patients who progress on aromatase inhibitors and often drive resistance to standard endocrine therapies. In the Phase 3 EMBER-3 trial, Inluriyo reduced the risk of progression or death by 38% compared with standard endocrine therapy. Its once-daily oral dosing allows patients to take treatment at home, offering both convenience and a meaningful new option for managing ESR1-mutated breast cancer.
A study is ongoing to evaluate imlunestrant in early ER+, HER2‑ breast cancer—learn more here.
- Journavx (suzetrigine)
Sponsor: Vertex Pharmaceuticals
Journavx is the first selective NaV1.8 inhibitor approved for acute pain, offering a non-opioid alternative for pain management. NaV1.8 is a sodium channel primarily involved in transmitting pain signals, and by selectively blocking it, suzetrigine can reduce pain without affecting the central nervous system in the way opioids do. In clinical studies, patients receiving Journavx reported significant pain relief with fewer systemic side effects compared to traditional opioid therapy. Journavx’s approval is considered a first-in-class milestone in acute pain therapy, establishing a new mechanism for non-opioid pain relief and setting the stage for safer alternatives to traditional analgesics.
As research continues to shape what comes next, ongoing clinical trials remain central to this progress. Explore current and upcoming breakthrough clinical trials at TrialX.com or sign up for our volunteer registry to stay notified about the latest study opportunities.


