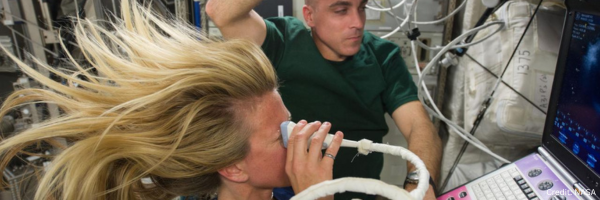
Supporting Health Research Data Collection for the Polaris Dawn Commercial Space Mission

Updated on September 18, 2024
The Polaris Dawn mission, a landmark commercial human spaceflight endeavor, launched into orbit on September 10, 2024 and the crew landed back safely on September 15th. We are excited to share that, in partnership with the Translational Research Institute for Space Health (TRISH), our team has played a pivotal role in developing the EXPAND App, which will ensure seamless pre-flight, in-flight and post-flight health research data collection for this mission. TrialX is enabling decentralized clinical research here on earth and in space with its cutting edge remote data collection platform. In addition to helping clinical trials investigators collect study data remotely, we are proud to support health research data collection for historic space missions like Polaris Dawn.
Watch the live launch here:
What is the Polaris Dawn Mission?
The first of up to three spaceflights planned in the Polaris program, the Polaris Dawn mission is a commercial space mission that aims to advance scientific research and enhance our understanding of human health both on Earth and in the vast expanse of space. This milestone mission pushed the boundaries of space exploration by testing a next-generation spacesuit during the first-ever commercial spacewalk, reaching the highest altitude in human spaceflight since the Apollo era, and evaluating a new communication system powered by Starlink. Read more about mission objectives here.
Watch the first-ever commercial spacewalk below:
Human Health Research & Data Collection During Polaris Dawn Mission
As part of this groundbreaking mission, the Polaris Dawn crew engaged in over 36 different scientific experiments, collecting invaluable health data to propel future space flights and improve health outcomes on Earth. The crew spent up to five days in orbit and our team is working tirelessly to enable crew collection of health research data seamlessly using integrated wearables and devices and nearly 30 surveys incorporated in the EXPAND App.

Thirty six health research projects from 31 partner institutions such as TRISH, SpaceX, University of Pennsylvania, NASA, Johns Hopkins, Hytro including others, were selected to be a part of this mission. The four-membered crew conducted a range of experiments to gather data on human health in space related to environmental health and hygiene, personality development, human body vitals, cognition, vision, motion sickness, decompression sickness, Spaceflight Associated Neuro-Ocular Syndrome (SANS), space radiation and more. In addition, the crew collected biological samples for contributing to a biobank for multi-omics analyses to understand the molecular changes that occur in space.
[To learn more about the biobank read our recent Nature publication “The Space Omics and Medical Atlas (SOMA) and International Astronaut Biobank,” that introduces the most extensive collection of data ever assembled for aerospace medicine and space biology.



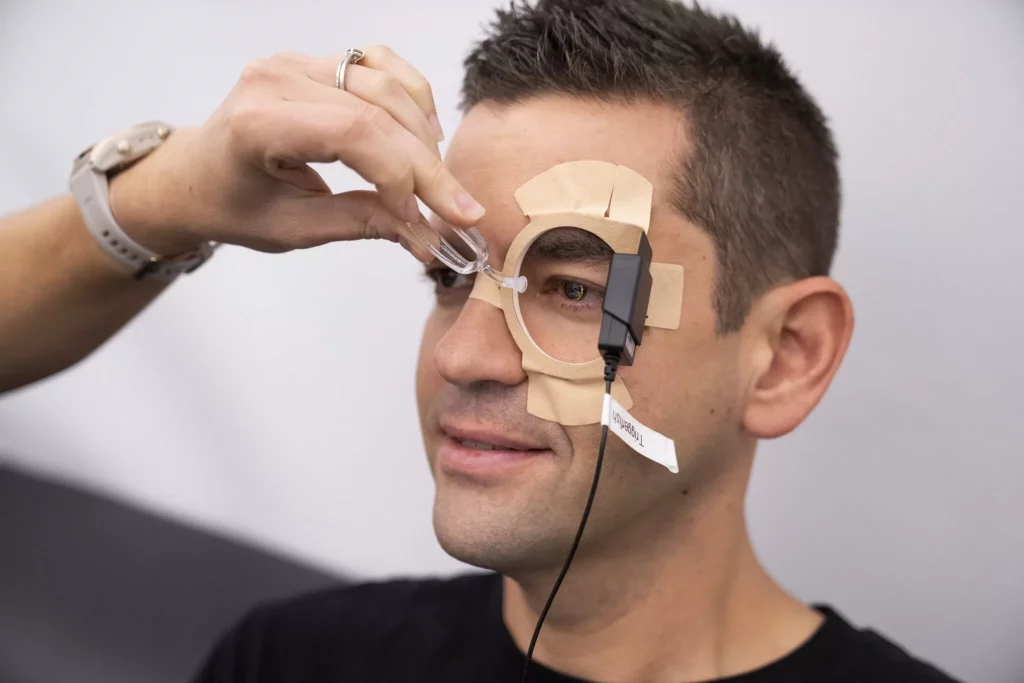
To gather data, the crew used many innovative wearables and devices such as Garmin smartwatch, a novel single-electrode sensor (BioButton, BioIntelliSense Inc.), smart contact lenses with tiny micro-sensors to measure pressure inside their eyes, a novel 3-D ultrasound device to build 3-D images of the structure of the eye, a CPR training device, a fiber optic camera and a miniaturized intelligent ultrasound device to take medical-grade images – to name a few.
You can visit the Polaris Dawn Science Research page to learn more about the experiments conducted during the Polaris Dawn mission.
TrialX’s Remote Data Collection Platform
TrialX’s Remote Data Collection Platform offers clinical trial investigators, participants and sites a way to conduct research remotely, reducing the need for live visits and capturing data remotely using mobile apps. It was developed as a platform that can ingest a variety of data types, including electronic health record summaries, wearable sensor data, diagnostic images, biospecimen analysis results and qualitative survey data.
In 2021, TrialX was selected by TRISH to build EXPAND DB – a first-of-its-kind database designed to collect research, flight and relevant clinical data from future commercial spaceflight missions. Leveraging our extensive experience in building clinical trial solutions, we were able to quickly customize the existing platform to meet the unique needs of the EXPAND program. The database supports a variety of data types across a multitude of individual research studies and currently houses data from subjects across six space missions – Inspiration 4, MS-20, Axiom-1, Axiom-2, Axiom-3 and Polaris Dawn. It equips space researchers to reuse and integrate research data across different research studies and unlock innovative actionable insights.
TrialX was also selected to be a part of another pioneering collaboration to develop systems to improve healthcare delivery and data management for spaceflight participants and researchers. Recently, we delivered a successful demo to NASA showcasing this innovation that aims to ensure prompt medical care for spaceflight participants in case of emergencies. It will enable them to collect health data offline and easily share it with crew in various space environments. In collaboration with our partners, our innovative work in space health research was recently published in Nature as two groundbreaking publications focusing on decentralized research in space missions, showcasing data collected using our cutting-edge technology platform.
Our remote data collection platform is the backbone of the EXPAND App, providing a robust and secure system for data collection, management and analysis. The platform integrates seamlessly with various health monitoring devices and sensors, ensures real-time data capture, implements top-tier security protocols and is designed to handle large volumes of data, including genomic data. We have successfully gathered required pre-flight data and in-flight data. Comprehensive post-flight health assessments data is in progress after the crew splashed down safely after five days in space.
We eagerly anticipate the valuable insights and advancements the Polaris Dawn Mission will bring. Stay tuned for more updates as we continue to support this extraordinary mission and push the boundaries of what’s possible in human spaceflight.
#PolarisDawn #Spacemission #Remotedatacollection #Clinicalresearch