Patient Centric Initiatives – What Methods Are Companies Implementing to Increase Patient Engagement?


Patient centricity has not always been the focus of the healthcare industry. About a decade back the role of the patient in healthcare product development was not considered to be that important, with healthcare companies focussing only on science and technology as the stepping stones of success.
However, over the last few years, patient involvement or patient engagement in healthcare development has begun to increasingly being recognized. The current healthcare industry does not only talk about patients only in terms of human subject protection in clinical trials, Nuremberg Code, Declaration of Helsinki, and Belmont Report, anymore, but goes a step forward to involve patients in various aspects of his health including drug development.
As patient perspective has becomes central to quality improvement, last year, in 2016, the Drugs Information Association (DIA) and TUFTS center for the study of drug development – performed a study collaboratively, to learn the patient-centric initiatives being taken up by the healthcare companies to increase patient involvement in drug development.

Jane Myles, Head, Operational Intelligence and Innovation, Genentech, A Member of the Roche Group; Hollie Schmidt, Vice President of Scientific Operations, Accelerated Cure Project and Barbara Zupancic, Director, Patient Recruitment, Worldwide Clinical Trials will talk about the key insights obtained from this study at the DIA2017 during June 18- June 22 in Chicago. This session will discuss examples of patient engagement initiatives, their implementation, the outcome after implementation and the ways they added value.
Key insights from the study
Patient engagement initiatives need not be necessarily costly to be effective. The key insights obtained from this research showed that low cost engagement initiatives generate highest returns. In addition, patient engagement initiatives improve trial performance with more positive study volunteer feedback and long term savings across drug development portfolio.
Organizations researching on diseases, regulatory agencies and private sector companies all have realized the importance of working around patients’s interests and are developing way to enhance patient involvement in their own health.
What are the efforts the companies are putting in?
So what are the efforts companies are putting in to involve patients in their processes. The DIA-Tufts CSDD study broadly divided the patient centric initiatives into 4 groups.
- Initiatives involving innovative partnerships
- Initiatives implemented using changes in protocol design
- Use of advances in technology
- Initiatives to facilitate study volunteer ease
Efforts that were most efficient in terms of ease of conduct and cost to conduct were:
- Advocacy group support and involvement
- Patient advisory panels and focus groups
- Social media/online engagements
- Direct-to-patient clinical trials/ Telemedicine
The initiatives that were implemented by most companies were:
- Use of patient organization landscape analysis tools
- Use of patient advisory boards
- Use of professional panels
Apart from these, other initiatives that was observed being used in the industry were:
- Patient counseling and education
- Adaptive trial designs and adaptive licensing
- Open design and crowdsourcing
- Home nursing networks and logistics assistance
- Apps for clinical data collection
- E-consent
- Digital medicine
- Gaming
What are the benefits that patient centricity efforts can provide?
Companies involved in healthcare are measuring their patient engagement efforts in terms of “ROE” – which expands to “Return on Engagement”. The DIA/TUFTS working group research shows that increasing patient- centric efforts lead to:
- Improvement in trial performance in terms of faster planning, approval and enrollment.
- Positive volunteer feedback
- Long term savings across drug development portfolio
Heatlhcare companies are bagging funding based on their efforts they are putting in towards patient engagement. For example, Braincheck raised 1.5 M for its Stanford University research based product consisting of iPad or desktop games to measure reaction time, visual processing, cognitive process, coordination, and memory. BrainCheck brings neuro-cognitive assessments to people who otherwise might not have access. Another company Outcome Health bagged $500 million in first round of funding itself, for patient education tablets.
However, we should be cautious of the fact that all patient engagement initiatives not necessarily enhance patient experience hand in hand.
TrialX’s efforts towards patient centricity
Dory – TrialX offers Dory – a patient facing clinical trial finder tool that helps patients navigate easily to clinical trial suitable for them. Dory also helps patients connect to their investigator or research team at a click of a button, thus facilitating patient engagement and speeding up recruitment. You can try the tool here: https://dory.trialx.com/ask/
iConnect – TrialX iConnect is specifically designed to facilitate patient-recruitment for academic medical centers, sponsors, and patient advocacy groups. iConnect allows investigators to improve online awareness of their trials, communicate with interested participants and monitor recruitment metrics.
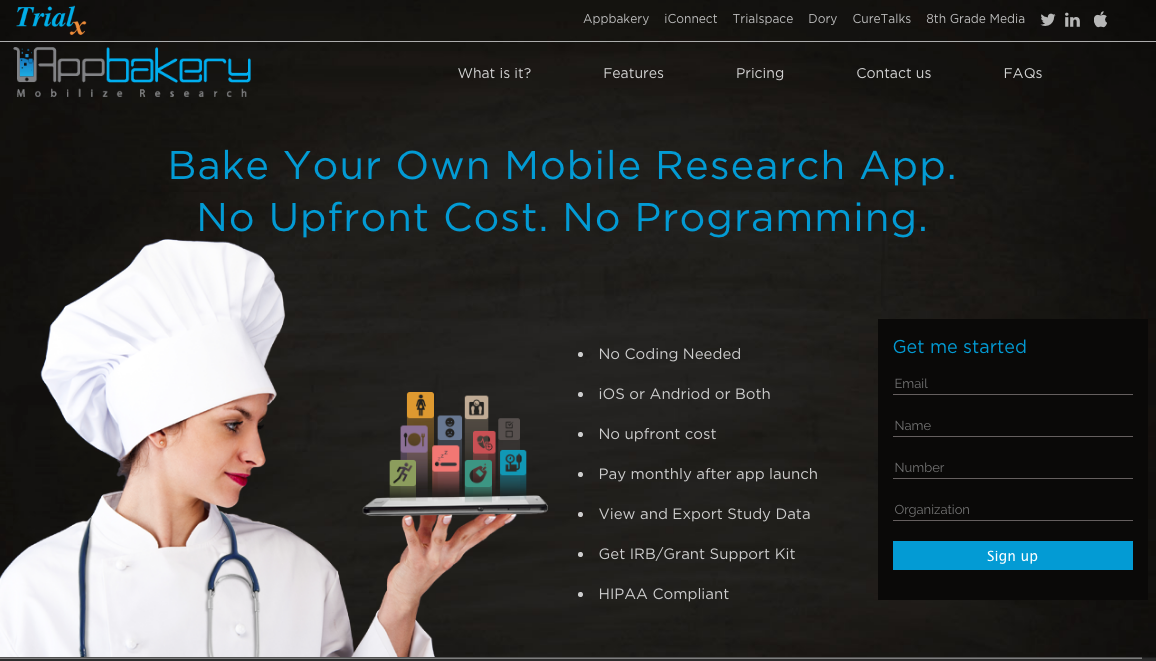
AppBakery – Appbakery is a revolutionary digital health platform using which researchers can build their own mobile research study apps which can collect patient generated health data to study metrics like lifestyle, psychosocial behavior, fitness, nutrition, sleep and more. It is a simple web wizard which can allow investigators to design their app and get their study running in no time.
The TrialX Nexus below describes other efforts that we have been putting in towards patient centricity and improvement in clinical trials success.

We are excited to learn more about the DIA/TUFTS working group research and what others are doing towards patient engagement at the #DIA2017 and would love to see you at booth number 2513 to share more about our efforts.