Obtaining Quality Data in Clinical Trials
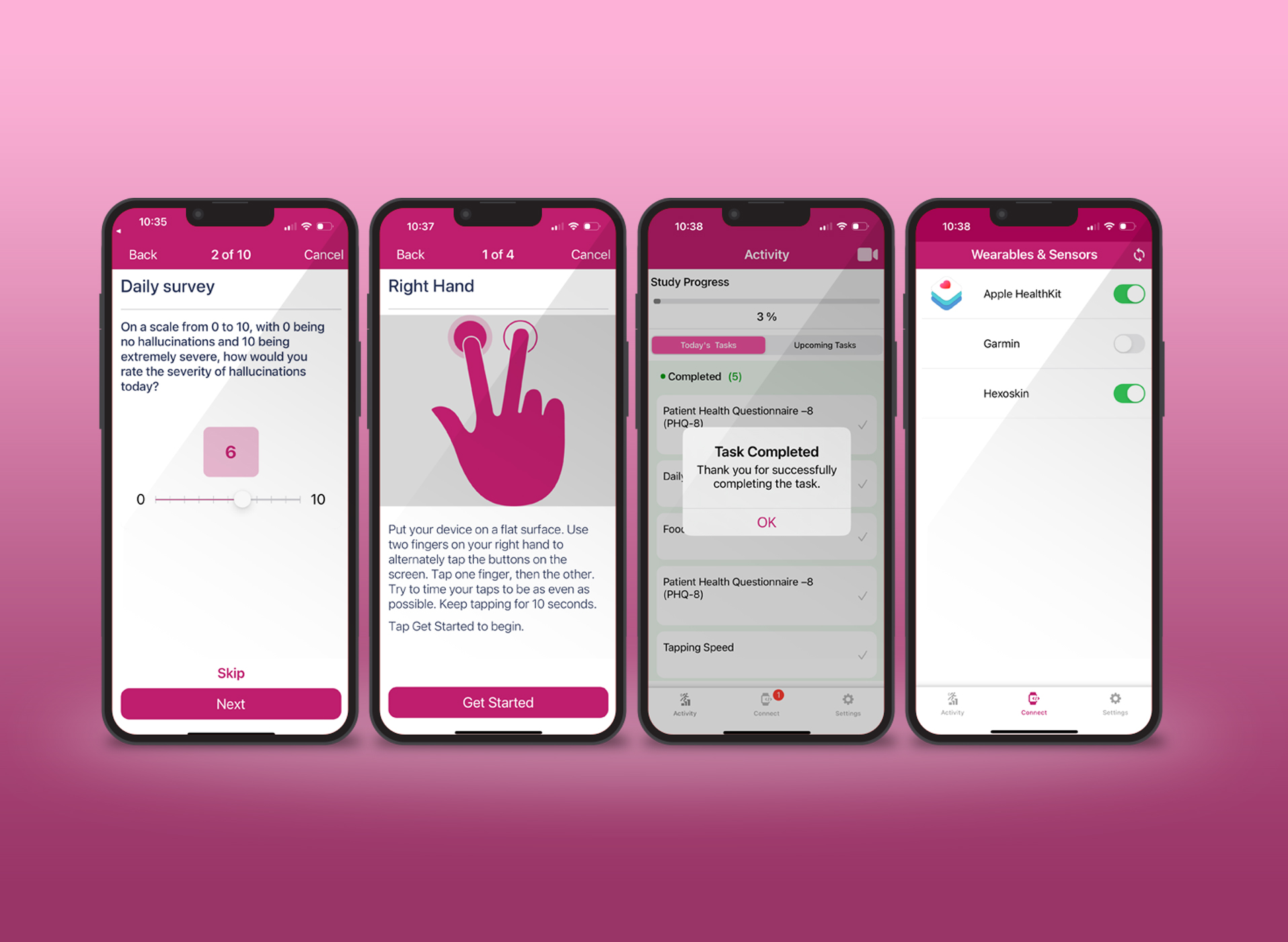
In the dynamic world of clinical research, the paradigm is shifting towards more sophisticated and patient-centric trial designs. This shift is driven by the need to streamline drug development processes, which are characterized by high costs and increasing complexity. As regulatory bodies like the FDA emphasize the importance of efficient and innovative approaches, the focus on quality data in clinical trials has never been more critical.
Why is Quality Data Important in Clinical Trials?
The Rising Costs and Complexity of Drug Development
The drug development landscape is marked by escalating costs, largely due to inefficient processes and complex regulatory requirements. This scenario is compounded by a slowdown in regulatory approvals, prompting a greater need for streamlined and cost-effective approaches in clinical trials. To adapt, sponsors are now required to present clean, high-quality data in easily shareable formats, while also focusing on reducing development costs.
The Importance of Quality Data in Regulatory Approvals and Drug Safety
Quality data is the cornerstone of regulatory approvals and patient safety. Regulators scrutinize clinical trial data to ensure that new treatments are both safe and effective. Moreover, accurate data is pivotal in identifying potential risks early in the drug development process, thereby safeguarding patient safety and upholding the scientific integrity of the trial. Here’s an insightful talk by the FDA on data quality – where CDER’s Deputy Center Director for Clinical Science, Robert J. Temple, M.D., shares case studies and FDA perspectives on why data quality is important in clinical trials.
How can we Enhance Clinical Trial Data Quality?
Innovative Clinical Trial Designs: Decentralized Trials, Synthetic Control Arms, Basket, and Umbrella Trials
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) – that aims to provide a unified standard for the European Union, Japan, the United States, Canada, and Switzerland to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions – released a new draft guideline, ICH E6(R3), on 19th May 2023. This guideline emphasizes the importance of appropriate clinical trial design to ensure that the trial objectives are met and that the data generated is reliable and robust.
Emerging clinical trial designs are reshaping how research is conducted. Decentralized trials, for example, allow for the collection of larger volumes of accurate data compared to traditional on-site Randomized Controlled Trials (RCTs). They offer more robust findings by including additional endpoints and capture data from a more diverse participant pool.
Synthetic control arms utilize real-world data in decision-making, leveraging information from sources like electronic health records (EHRs) and administrative claims data. Basket and umbrella trials, often employed in rare disease research, test a single intervention against multiple indications or multiple interventions for a single indication, respectively. These innovative designs allow for more flexibility and efficiency compared to standard RCTs.
Leveraging Digital Technologies: AI, ML, and Big Data Analytics
The successful management of complex trials increasingly relies on digital technologies. AI and ML are creating efficiencies in data management, while big data analytics are crucial in deriving meaningful insights from vast amounts of trial data. These technologies facilitate sophisticated data capture and management, enhancing the interoperability of clinical trial data. The WHO and FDA are at the forefront of exploring and advocating for responsible AI use in drug development. These organizations emphasize the establishment of principles, standards, and best practices for AI and ML in this field, aiming to make drug development more efficient and effective.
Regulatory Guidance for Clinical Data Quality
Emphasizing Electronic Data and Ethical AI Use
Regulatory bodies are actively issuing guidance on the use of digital technologies in clinical trials. For instance, the FDA’s recent draft guidance on decentralized trials and the ICH’s new guidelines on data endpoints reflect a growing regulatory emphasis on electronic data quality and the ethical use of AI in drug development.
Minimizing Data Complexity
In line with reducing complexity in clinical trials, the ICH’s new guidance stresses the importance of limiting trial data collection to essential information. This approach aims to minimize the costs and risks associated with storing excessive data, thereby streamlining the trial process.
Preparing for the Future: Adapting Modern Technology and Innovations
As precision medicine and AI-designed clinical trials become mainstream, it’s imperative for sponsors, CROs and clinical research stakeholders to adapt their clinical research processes. By doing so, they can ensure the generation of high-quality data and streamline trial processes for developing safer and effective treatments – as regulators continue to push for increased efficiency, more patient-centric approaches, and expanded reliance on eSource in clinical research.
TrialX Solutions: Enhancing Efficiency in Clinical Trials and Improving Data Quality
TrialX’s advanced technologies and platforms are designed to support innovative trial designs, ensuring efficient data management and quality control. By integrating cutting-edge digital tools that facilitate decentralized clinical trials, TrialX helps in streamlining the data collection process, maintaining data integrity, and ensuring compliance with regulatory standards.