Mobile in Clinical Trials: Challenges and Barriers in Adoption
According to a 2016 industry survey by FierceBiotech, more pharmaceutical companies are currently planning or piloting the use of mHealth (using devices such as sensors or wearables), in their clinical trials, than actually conducting trials using the technology. The survey included 108 respondents from a list of trial sponsors and CROs. It found that of the trials in which devices were actually being used, two-thirds were regulatory-approved devices, while another one third was using consumer, activity, or smartphones.
The use of mHealth technology presents a significant change for the industry and comes with costs attached.
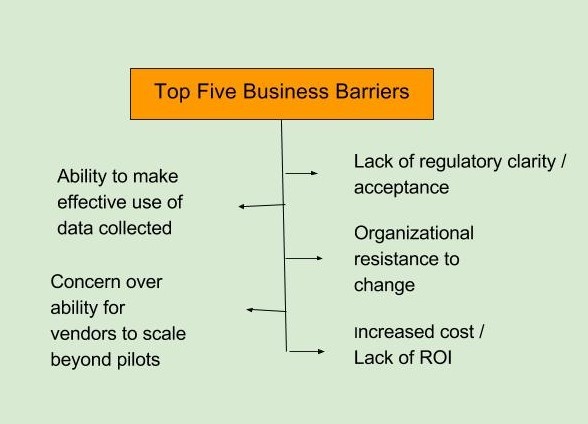
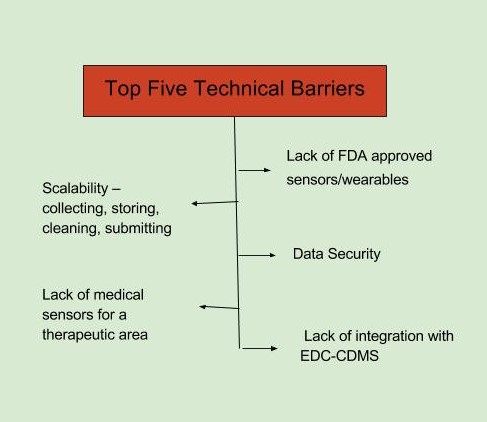
The top barriers to adoption of mHealth technology, as identified in the FierceBiotech survey, fall in two broad categories, business and technical. The top business-related barriers are seen as a lack of regulatory clarity and acceptance and an organizational resistance to change. The top technical barriers are defined as a lack of FDA-approved sensors/wearables and data security (FierceBiotech survey, July 2016 n=108).
Key Challenges of mHealth Adoption in the Clinical Trials Industry
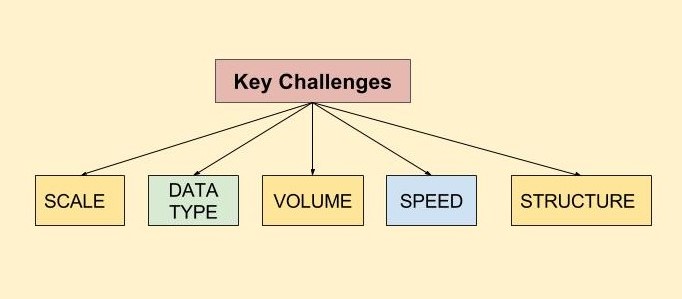
With the advent of mhealth, the clinical trials industry faces the challenges of storing, aggregating, correlating, and analyzing vast volumes of data from various mhealth technologies to find what is relevant and clinically meaningful.
Data can now be produced from multiple sources, in structured or unstructured formats, and in varying volumes, with different standards attached. This data may come from mobile applications, medical devices, clinical lab test results, patient e-diaries (covering a patient’s physical or emotional responses or describing an adverse event), discharge summaries and clinician notes, electronic medical records, other free-text notes, and/or video/ imaging information.
The volume of data varies greatly depending on the frequency of reporting. Data collection frequency can vary from once or twice a day (a drug ingestion event or a blood pressure (BP) measurement), to a high-frequency basis. Data types can include such information as activity or sleep data, blood pressure monitoring, continuous heart-rate every few seconds, or other physical movements or physiological measures. For just one patient, per day, there is the potential to create a huge volume of multiple types of real world data.
By collecting the data in a real life situation, there is the opportunity to gather far richer datasets than are typically available in periodic, six weekly, physician appointments. Self-measurement of daily BP not only provides more data, but also gives a realistic picture of the true, normal BP of the patient, rather than the potentially inflated BP triggered by white coat syndrome in the physician’s clinic. Collecting more frequent data points enables biostatisticians to increase the power of their analyses and helps drive a stronger conclusion, as to whether the new drug is effective and safe.
This richer data source provides huge opportunities and a few challenges. For example, simply measuring pulse every second produces 86,400 pulse measurements a day. So, for a two-year, 500-subject clinical trial, that would yield 31 billion data points. Exploring, visualizing, and analyzing such large data volumes is relatively new for clinical research. But, so-called, big data technologies exist across a wide array of industries to handle these volumes. By correlating this data with other clinical data in the trial and with external data, there exists the opportunity and possibility of new discoveries.
Further, advances in machine learning and artificial intelligence (AI) can help to identify new correlations and derive new potential hypotheses and patterns. While it is likely to be many years before AI becomes main stream in clinical research, the opportunity for such techniques, combined with ever-increasing, lower-cost, computing power, enable clinical data scientists to explore new pathways and relationships that were impossible to consider with the traditional, discrete, tiny datasets. The availability of more data leads to presumably better insight and greater potential to discover any additional drawbacks that the drug or therapy might cause.
A drug that looked effective and safe using the old paradigm might be exposed as unsafe and ineffective through analysis of such real world data. This insight must be recognized by the industry as a positive and not used as a barrier to stifle innovation. Real world evidence must be leveraged as an enhanced opportunity to drive development of new, safe, and effective therapies. The ability to gather large and diverse data directly from the patient and correlate them with external data is just the start of a new digital journey. The necessary technologies exist today in other data-rich industries.
Protecting your Patient’s Privacy in a Mobile World
With the rise of mHealth, patients are becoming ever more actively involved in their own care. But one area where this has significant implications is patient privacy – if contact with patients is more flexible and frequent, can it always be kept confidential?
Data gathered by mhealth applications may be accessible to the patient, but it may also be shared with others such as physicians; family members or carers; or scientific researchers. There are also considerations about the way data is stored and processed, creating a need for strong security and audit trails.
The key challenges include managing who has access to a health record – whether a patient or a healthcare professional – and ensuring that only secure, approved devices and applications can access data. Assurances of anonymity are a top priority for gaining the trust of patients, but location data in particular is a challenge for anonymity in mHealth applications. Ultimately, to make strong progress with mHealth, policymakers must draft the laws, regulations, and standards needed to protect patient privacy, and enforcement agencies must ensure these are implemented. Involvement by technology developers will also be essential. Above all, the patient’s needs should always remain at the center – remembering that the free flow of data is vital for improving care, as long as privacy is protected.
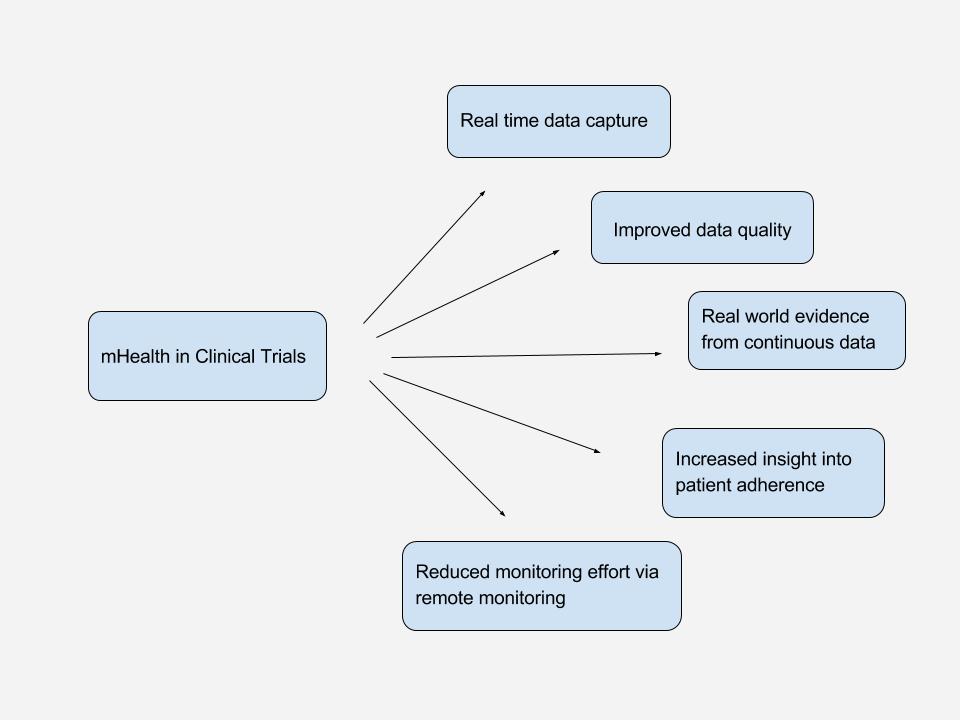
mHealth in Clinical Trials
The World Health Organization defines mHealth as a component of e-health, and “a medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants (PDAs), and other wireless devices”.
While the US Food and Drug Administration (FDA) had offered guidance only around “mobile medical apps,” previously, the FDA has launched its “Digital Health” initiative.5 In the overview, the agency defines digital health as: “The broad scope of digital health includes categories such as mobile health (mHealth), health information technology (IT), wearable devices, telehealth and telemedicine, and personalized medicine.”
Even though there are many, varying definitions, it is clear that mHealth has the potential to connect patients to researchers, care providers and deliver data from real-world.