How to Participate in a Clinical Trial: A Simple Guide

Behind every medical breakthrough is someone who said yes to research. Clinical trials don’t just test new treatments—they open the door to progress in care, diagnosis, and prevention. But for those considering participation, the journey often begins with questions.
From understanding safety to figuring out how to take the first step, the path can feel unfamiliar. If you’re exploring options, finding a clinical trial that aligns with your health needs and preferences is an important first step.
This blog outlines how clinical trials work and what steps are typically involved for those considering participation.
Common Concerns About Participation
Hesitation around clinical trial participation is understandable. Common concerns include:
- Fear of being treated as a “test subject”
- Worries about safety, side effects, or unknown risks
- Uncertainty about cost or insurance coverage
- Concerns about disrupting current treatment
- Previous negative experiences within the healthcare system
It is important to know that:
- Participation is voluntary
- Consent is required before any procedures begin
- Participants may leave at any point without penalty
- Clinical trials follow strict safety and ethical standards
Eligibility to Participate
Each clinical trial has its own eligibility criteria, designed to ensure safety and collect reliable data. Criteria may include:
- Age
- Gender
- Diagnosis and disease stage
- Medical history
- Current medications or treatments
- Overall health
- Biomarkers or genetic factors (in some studies)
Eligibility is confirmed through multiple screening processes conducted by the study team.
What to Expect: The Participation Process
1. Initial Interest
It’s important to find a clinical trial that’s relevant to you. Many people first hear about studies through their healthcare provider, from friends or advocacy/support groups, or by searching online. Although registries like clinicaltrials.gov list out information on clinical trials, the presentation of information is difficult for many to navigate and understand. Platforms like TrialX’s AI-powered matching tool make it easier to explore trials that align with your specific condition and needs.
Once a trial seems relevant to you, the next step is usually a brief eligibility check.
2. Pre-Screening
Before diving into all the study details, most trial teams ask a few short questions about your age, health, location, and medical history to see if the study might be a good fit.
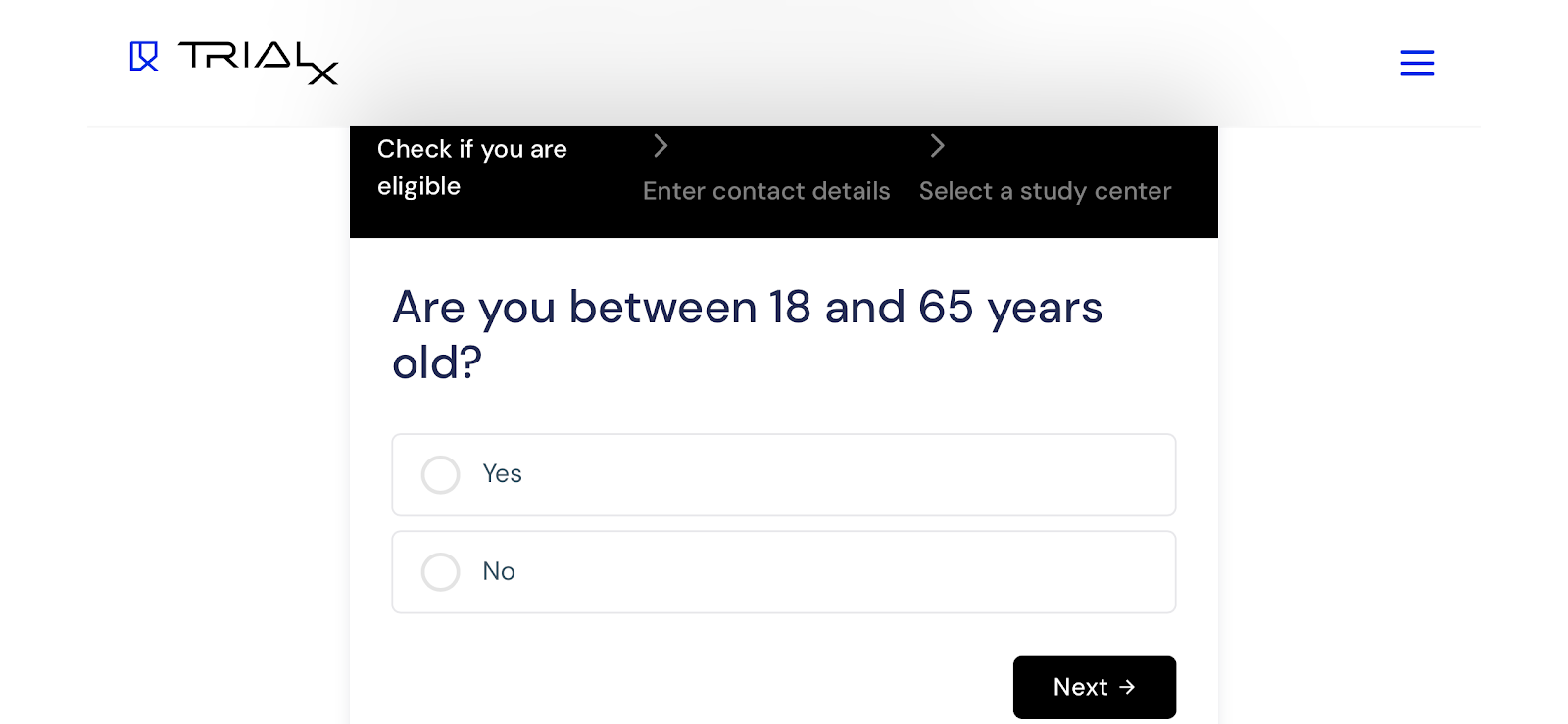
With our interactive pre-screener, you can answer these in just a few clicks and quickly find out if you may qualify. If you do, the trial team will follow up with more details and answer any questions.
3. Contact from the Research Team
A clinical research coordinator, nurse, or investigator may reach out to you to discuss:
- Study purpose
- What participation involves
- Time commitments
- Any known risks or benefits
At this stage, no decisions are required. This step is simply to provide information and answer questions.
4. Informed Consent Process
Informed consent is a required and critical part of ethical research. Participants receive a document called the Informed Consent Form (ICF) that clearly explains:
- The study’s purpose
- What participation includes (visits, treatments, tests, etc.)
- Possible risks and benefits
- Alternatives to participation
- How data will be handled and protected
- The right to withdraw at any time
Time is provided to review the ICF, ask questions, and consult with trusted individuals, such as family members or healthcare providers. Signing the ICF does not obligate anyone to complete the trial, but it is required to begin the next steps.
5. Screening Visit
After consent, a formal screening visit is often conducted to confirm eligibility. This may include:
- Blood tests
- Physical examinations
- Imaging scans
- Review of medical records
Some individuals may not qualify for final enrollment based on test results or detailed criteria. In such cases, other trial options may be suggested. With our virtual waiting room, these individuals can remain connected to the study team and be re-contacted if their eligibility changes.
6. Enrollment and Participation
Those who meet all requirements and choose to proceed are officially enrolled in the trial. Depending on the study, participation may include:
- Taking a medication or receiving a procedure
- Attending regular check-ins or follow-up appointments
- Completing electronic diaries or questionnaires
- Communicating regularly with the study team
Participants are closely monitored for any side effects or concerns. The length and intensity of involvement vary by study, and participants are free to exit the study at any time.
Questions to Ask Before Joining a Clinical Trial
Here are some helpful questions to consider:
- What is the purpose of the study?
- What phase is the trial in (Phase I, II, III)?
- What are the possible risks and side effects?
- Will I receive the treatment, or could I get a placebo?
- How often do I need to visit, and where?
- Are there any costs I should expect?
- Can I still see my regular doctor?
- How is my personal data kept safe?
- What happens after the study ends?
Explore Trials Near You
Joining a clinical trial is a personal choice—one that deserves clear, accessible information and reliable support. Whether you are just beginning to explore or actively searching for opportunities, our AI-powered matching platform helps individuals find studies that align with their health needs and preferences.
To make clinical trial information easier to understand, TrialX offers content in simplified language at 5th and 8th-grade reading levels. Multilingual access is also available to support diverse communities and help more people make informed decisions about participation. We are supporting several advocacy groups—including the Michael J. Fox Foundation, the Sickle Cell Disease Association of America, Let’s Win Pancreatic Cancer, Reflections Initiative, Beyond Celiac, and ALSN – to help the patient communities have faster and better access to relevant clinical trials through our AI-powered clinical trial finders.
If you are interested in participating in a clinical trial, you can explore trials here or sign up for our Volunteer Registry to get notifications on relevant clinical trials.


