How Far Do Patients Travel to Participate in Clinical Trials?
“Being in clinical trials required a lot of travel, which was costly and tough on my body.”
– Katie Doble, cancer trial participant in Colorado
For Katie, joining a clinical trial meant leaving her home state and relocating to New York City for several weeks. The treatments offered her hope, but the travel added another layer of challenge—long distances, added expenses, and time away from her normal life.
Katie’s experience reflects what many participants face. Data shows that in cancer clinical trials, patients travel a median of 25.8 miles each way, with some traveling over 100 miles per visit. Across all trial types, the average distance is even higher—around 67 miles one way, and for rare diseases, the burden can reach 135 miles.
The Travel Gap in Clinical Trial Access
While travel affects nearly all participants, the challenges can be more significant for certain groups, depending on their condition, socioeconomic status, where they live, or the kind of care they need.
1. Rural and Underserved Communities
For people living outside big cities, reaching a trial site can be a major challenge. An NIH study found that 74% of rural patients had to travel more than 50 miles to attend a trial, compared with only 16% of urban patients. Many rely on long drives, private transport, or help from family, and when trials require frequent visits, the burden grows heavier—often discouraging people from taking part.
2. Rare Disease Patients
Trials for rare diseases are usually offered only at a few specialized hospitals. Diagnosis itself often takes years, as symptoms are frequently mistaken for more common conditions. By the time a diagnosis is confirmed, patients may discover that only a handful of trials exist—often concentrated at specialized centers far from home.
Travel demands for rare cancer patients are especially high. While average travel times are similar to those of common cancers (about 45 vs. 43 minutes), patients at the farthest distances can spend nearly an extra hour each way. Over 13% of rare cancer patients travel more than two hours one way, compared with 8% of common cancer patients.
Even after finding a study, participation brings new challenges. Many rare disease patients live with limited energy, need ongoing care, or must carry essential medications and equipment while traveling. Extended stays away from home can also be stressful, disrupting routines and separating patients from their support systems.
This shows that it’s not just about how many miles people travel—time, frequency of visits, and type of treatment all matter in whether patients can realistically join a trial.
3. Financial Strain from Indirect Costs
Research from the Stanford Cancer Institute highlights the financial challenges faced by low-income participants in clinical trials. Even when treatment itself is provided at no cost, participants often encounter hidden expenses such as transportation, lodging, meals, parking, and childcare. The study found that travel reimbursement programs are not always sufficient or timely, leaving participants to cover some costs out of pocket. These hidden costs can affect participants’ decision to stay in a trial.
4. Health and Mobility Limitations
Some patients struggle because of their health. Fatigue, advanced illness, or mobility problems make travel exhausting. As one participant shared on HealthTalk.org,
“Well, at the start, it meant my husband had to have a day off each time I went, because I wasn’t well enough to drive. So every three weeks, my husband would have to take a day off… it impacted more financially than anything else.”
For people already dealing with serious illness, these challenges can make participation feel out of reach.
Comparing Travel Burdens Across Therapeutic Areas
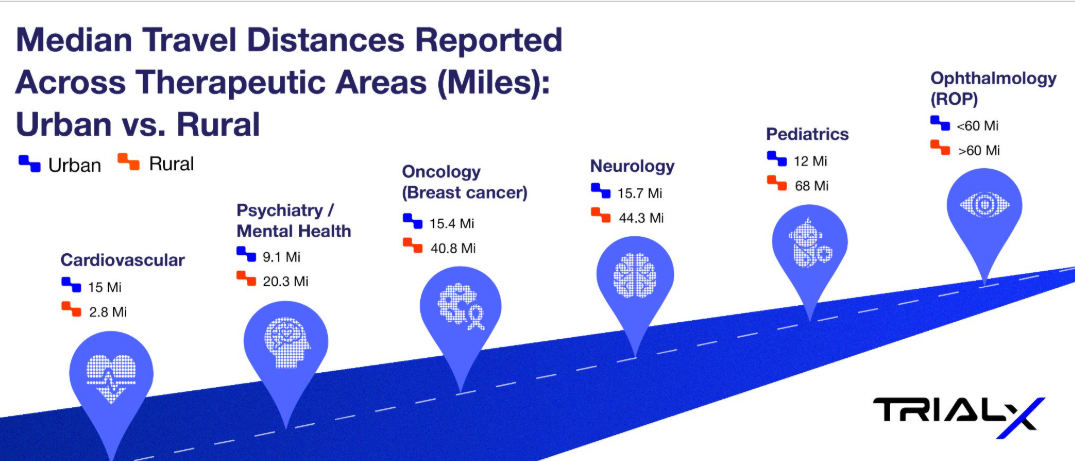
Research shows that travel distances vary widely depending on the therapeutic area and whether patients live in urban or rural settings. The infographic below highlights median distances patients need to travel to access clinical trials across therapeutic areas – as reported in different studies (reference provided in the table below).
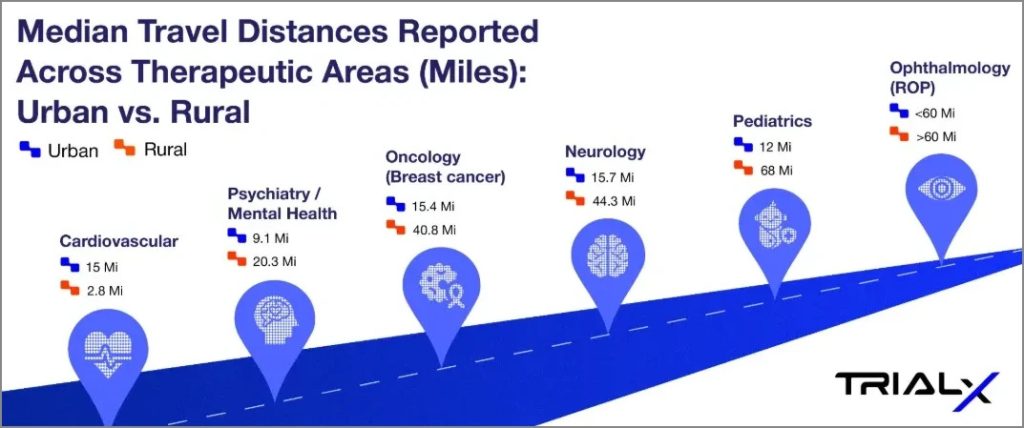
*Median Travel Distances Reported Across Therapeutic Areas: Urban vs. Rural
| Therapeutic area | Urban | Rural | Source | Context |
| Cardiovascular | 15.0 miles | 2.8 miles | Reference | Cardiovascular clinical trial sites are heavily concentrated in urban areas, with only 5% located in rural ZIP codes. While urban residents traveled a median of 4.5 km, rural residents faced a much longer distance of 24.2 km—over five times farther. |
| Neurology | 15.7 miles | 44.3 miles | Reference | The study analyzed 563,216 Medicare beneficiaries in 2018 who had at least one outpatient neurologist visit. Rural patients had a median one-way travel distance of 44.3 miles compared to 15.7 miles for urban patients, and 44.7% of rural residents traveled ≥50 miles versus only 11.2% of urban residents. |
| Psychiatry / Mental Health | 9.1 miles | 20.3 miles | Reference | In a U.S. study of 3,870 patients with depression, rural residents lived more than twice as far from their primary care clinics as urban residents. Greater travel distance was associated with slightly higher remission rates at 6 months and lower persistent depressive symptoms, highlighting geographic disparities in access to mental health care. |
| Oncology (Breast cancer) | 15.4 miles | 40.8 miles | Reference | Rural breast cancer patients traveled nearly 3 times farther for radiation therapy than urban patients (40.8 vs 15.4 miles), with the nearest facility also over 4 times farther away (21.9 vs 4.8 miles). These findings underscore how rurality—not disease severity—drives access burdens in oncology care. |
| Ophthalmology (ROP) | <60 miles | >60 miles | Reference 1 Reference 2 | Residents in rural and low-income areas faced significantly greater travel burdens to reach ROP clinical trial sites, often exceeding 60 miles or 60 minutes |
| Pediatrics | 12 miles | 68 miles | Reference | This study compared demographic and clinical characteristics of rural vs nonrural children hospitalized in children’s hospitals. It specifically looked at travel distance, socioeconomic status, and clinical complexity. |
How Technology Brings Research Closer to Home
The good news is that new approaches to clinical research are reducing the need for long commutes, making trials more inclusive and manageable.
- AI-Powered Trial Matching
One of the biggest barriers patients face isn’t just the travel itself—it’s knowing where to begin. While ClinicalTrials.gov lists thousands of studies, the information is often full of medical jargon, lengthy descriptions, and complex eligibility rules that can be difficult to navigate. For someone already coping with illness, finding a suitable clinical trial can feel out of reach.
TrialX’s AI-powered matching system helps simplify this process by highlighting trial opportunities that best fit a patient’s condition and location—including options that may be fully virtual or hybrid, reducing or even eliminating the need for long-distance travel.
Many advocacy groups, such as the Michael J. Fox Foundation, Let’s Win Pancreatic Cancer, Sickle Cell Disease Association of America, Reflections Initiative, Beyond Celiac, ALS Network, among others, have trusted this tool to make trial access more inclusive for their patient communities.
- Decentralized Clinical Trials
Decentralized clinical trials (DCTs) are designed to bring research closer to participants by using technology and remote methods to minimize or eliminate the need for travel to traditional trial sites.
Some studies are fully virtual, where everything from recruitment to follow-up takes place online. Others follow a hybrid model, combining remote tools with occasional in-person visits. When clinic visits are needed, participants may go to local labs, receive medications from visiting nurses, or attend brief check-ins at accessible locations.
TrialX’s Remote Data Collection (RDC) system plays a key role in enabling this model. For example, it supports:
- Pre-screening & Informed Consent – Simplifies recruitment and ensures compliance by automating pre-screening, managing eligibility checks, and capturing consent remotely.
- Data Capture and Monitoring – Integrates wearables, telehealth platforms, and electronic patient-reported outcomes (ePROs) to help participants track symptoms, consult clinicians via video calls, and record health data in real time.
- Ongoing Engagement- Enables timely reminders, notifications, and data validation to ensure compliance and enhance retention throughout the trial.
The SAYSTOP Study App, developed by TrialX for antibiotic research, is one example of how this works in practice. The app allows participants to log symptoms, track medications, and receive reminders from home, while researchers capture accurate data through integrated real-time analytics.
Ultimately, DCTs and virtual clinical trials are making participation more convenient, inclusive, and manageable, allowing patients to stay engaged throughout the study.
Frequently Asked Participant Questions
- Do patients get paid for participating in clinical trials?
Participants may receive compensation, but it is usually meant to offset costs like time, travel, or inconvenience rather than serve as income.
- Who covers patient expenses in a clinical trial?
It varies. Some trials cover travel, lodging, or other expenses, while others may not. Patients should ask the trial team for details before enrolling.
- How can patients find clinical trials closer to home?
Resources like the TrialX Clinical Trial Finder let patients search by condition and location, making it easier to identify studies that require less travel.
- Can patients participate if they do not live near a clinical trial site or face travel limitations?
Increasingly, yes. Decentralized and virtual trial options allow many participants to join remotely, using telehealth, local labs, and home delivery of medications.
Interested in Exploring Clinical Trials Near You..
Our TrialX’s AI-powered clinical trial matching system makes it easy to find relevant clinical trials nearby, simply by entering basic information such as condition and location. Patients and caregivers can also sign up for our volunteer registry to stay informed about studies that match their health profile.