Electronic Patient Reported Outcomes (e-PRO): Role in Patient Engagement, Recruitment and Retention
There is a growing realization for patient-centered healthcare system. The outcomes of a clinical intervention obtained by the patient i.e. patient-reported outcomes (PROs) may be of more importance in future than any other outcomes like clinical, physiological or caregiver-reported. As per studies, enhanced treatment adherence and outcomes can be obtained by giving attention to patient feedback on healthcare outcomes and patient behavior change. As per US-FDA,
A PRO is any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.
When the outcomes of the patients are collected by using electronic methods like electronic diaries, computers, telephones etc, it is e-PRO or electronic PRO. The use of e-PRO are found to be beneficial than paper based PRO.
Image courtesy: https://www.research.nhg.com.sg
ePRO Advantage
- Avoid data entry errors
- Immediate access to data
- Enabled to trigger alerts/notifications
- Reduce missing information
- Increase patient’s willingness to report sensitive information
- Real-time tracking of survey compliance
ePRO Barriers
- Increased expense
- Human error
- Limited time for patient-training and infrastructure study site
- Cultural resistance for administration of electronic instrument
ePROs in a Clinical Trial Setting
Patient recruitment and retention has always been a major cause of delay and expense in clinical trials. Almost 80% of clinical trials fail to meet their enrolment timelines, and nearly 50% of trial sites enroll only one or zero patients. Patient recruitment activities represent more than 25% of overall trial costs — according to a study by the U.S. Department of Health and Human Services (HHS), an average of $805,785 is spent on recruitment from Phase 1 through Phase 4.
The patient-centricity movement in clinical trials recognizes that if patients are supported throughout a study, higher levels of compliance and retention can be achieved. Continuous engagement with patients throughout their clinical trial experience, and simplifying methods of data capture and reporting are now seen as vital to clinical study success.
The rise of electronic clinical outcome assessments (eCOAs), and specifically electronic patient reported outcome (ePRO) tools implemented through everyday mobile technology, has transformed how patients can be engaged throughout a clinical trial.
Using these tools, pharmaceutical companies can now design studies to include relevant, standardized, and tailored communication plans, delivered through text messaging, email, and/or in-app notifications. As such, patients can be prompted to take medication, record health data, and attend site visits. Plus, sites can follow up with patients if data isn’t being entered in the correct manner, rather than waiting for their next site visit.
BYOD (bring your own device) trials, especially in the early development stage, offers true scalability for eCOA and has the potential to greatly reduce the cost and logistical complications of providing and maintaining traditional ePRO devices for trial participants.
The high data collection rate of ePRO is another very attractive feature. A large part of this is due to the relative ease-of-use of ePRO interfaces. Because of the decreased steps required for trial participants to provide confidential feedback (with one click, a patient is directed to a questionnaire), individuals may be more willing to provide honest information they may not otherwise be comfortable sharing with clinical staff members.
Further, ePRO questionnaires can be sent and reviewed in real-time, meaning the potential for lost, missing or noncompliant data is significantly reduced. Because data is collected in real-time, ePRO can help also help facilitate interim data analysis when required according to study protocol. Finally, at the end of a study, data already exists in the study database, which further speeds study close-out.
Another area in which ePRO capabilities assist with study efficiencies relates to cost and decreasing overall study expenditures. Consider that in 2012, it was estimated that the expense of bringing a drug or device to the marketplace ranged from $800M to $2B. Depending on the product under investigation, clinical studies accounted for 50%, to as much as 65%, of that cost. ePRO assists in decreasing costs by eliminating or lessening the frequency with which subjects are required to visit a healthcare facility or to mail in outcomes information. This also creates greater convenience for subjects and assists greatly with compliance and participation.
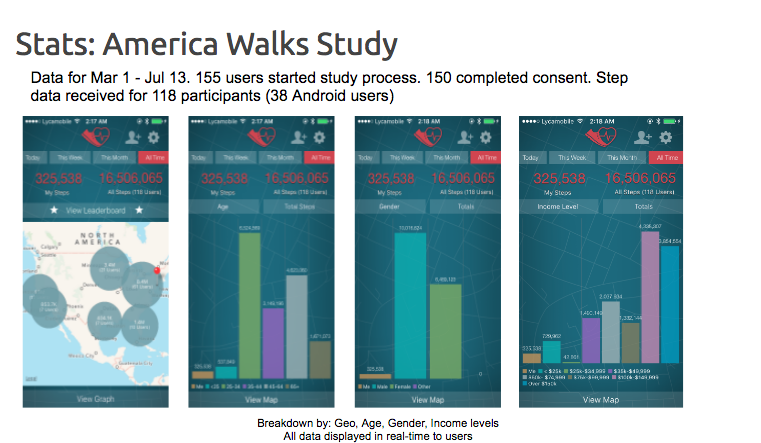
Case Study: America Walks Study
In March 2016, TrialX launched America Walks Study (AWS) App, a much smaller study to investigate the walking behavior of Americans, and to find out how much they are actually walking compared to their perception of how much they walk, and also compared to their buddy walkers who downloaded the app. Data collected from March 1 to July 13, shows that 155 users downloaded the study of which 150 completed the e-consent process and the initial demographic survey.
The AWS app provided participants with a dashboard with real-time data displayed to users, where they can see their number of steps walked along with their ranking in their state as well as the overall ranking.
This feature of researchkit study apps that allows immediate data sharing, enhances user experience and motivates users to continue to participate in the study, in other words, helps increase adherence. 193 participants from almost 32 states across America walked more than 24M steps. The walk data collected from the participants can be sliced and diced based on age, gender, geographical region, sex, and income levels. The study will be presented in the Poster Presentation category in the upcoming AMIA conference in November 2017.