DIA 2024 Highlights: TrialX’s Cutting-Edge Clinical Trial Management Solutions & Heartwarming Clinical Research Songs Steal the Spotlight!

The DIA 2024 conference concluded, and we are thrilled to share the highlights of our participation. A prominent event – attended by 4K+ people and featuring 220 sessions with more than 500 speakers across 13 engaging content tracks – DIA 2024 witnessed TrialX making waves with its innovative solutions and engaging musical offerings at Booth #1033. Although DIA 2024 seemed slower than previous years, we had a pretty good number of visitors at the TrialX booth, thanks to our “Songs for the Unsung Hero” offering! This initiative made people laugh and the emotions poured out. It also allowed us an opportunity to share our expertise in addressing challenges such as patient recruitment and remote data collection within the clinical research landscape.
Innovations and Insights at DIA2024
The Global Annual Meeting of DIA 2024 saw an exploration of transformative trends and imperative shifts within the biopharmaceutical industry. The event kicked off with a focus on embracing innovation and risk-taking to propel breakthroughs in patient outcomes. Key themes encompassed the evolving role of artificial intelligence (AI) in drug development, the increasing emphasis on patient engagement, and the necessity for regulatory agencies to adapt swiftly to cutting-edge scientific advancements. Generative AI seemed to be top of mind with companies looking for ways to incorporate AI into their business. TrialX led the way with the launch of our AI-powered clinical trial finder tool.
Thought leaders, including Peter Marks, Director, Center for Biologics Evaluation and Research from the FDA and Monica Eason of Genentech, emphasized the need for bold initiatives in diversity, patient-centric approaches, and regulatory evolution to drive industry growth and enhance healthcare delivery. The sessions underscored the critical importance of collaboration, globalization, and leveraging technology to accelerate the development of innovative therapies for global populations.
As DIA 2024 progressed, the conversations deepened on patient advocacy and the crucial need to amplify the patient voice. The Paladin (Patient Advocacy Leaders and Drug Development Industry Network) discussion grabbed a lot of attention. The goal is to build an expansive network of advocacy groups and industry partners that can tap into all therapeutic areas including rare diseases, and to expand year after year, getting real-world experience through reflections.
In addition, discussions highlighted inclusive trial design, the imperative for regulatory convergence to advance pharmaceutical innovation, the need to broaden diversity in clinical trials, and harness technology to democratize healthcare access. Respected figures from the USFDA, EMA including patient advocates such as Ella Balasa brought attention to the power of patient-centric practices, the expansion of trial inclusivity, and the role of regulators in fostering disruptive approaches for better medical solutions. The event culminated in a focus on AI integration, regulatory alignment, and streamlined clinical trial practices, showcasing a commitment to advancing knowledge, collaboration, and pragmatic approaches to usher in a new era of patient-focused, innovative healthcare solutions.
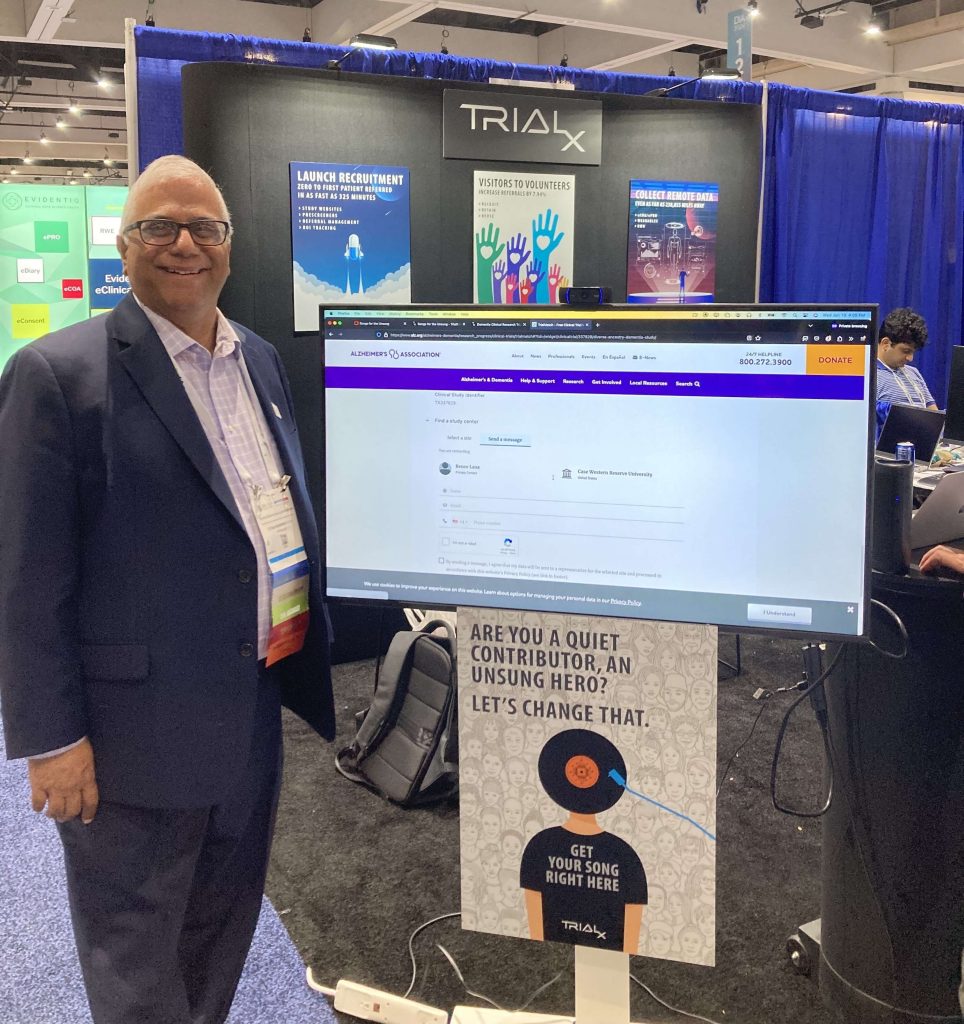
TrialX’s Unique Offerings at DIA2024 Booth #1033
At TrialX, we are committed to overcoming hurdles in clinical research with innovative solutions that transcend boundaries—both on Earth and in space. At the DIA2024, our CTO, Dr. Chintan Patel, and CSO, Paul Donnelly, demonstrated how our enterprise patient recruitment platform and remote data collection platform are revolutionizing clinical trials, emphasizing efficiency and inclusivity for both patients and investigators. Moreover, visitors at the booth had the opportunity to sign up for a personalized song in their name, adding a touch of creativity and fun to their DIA 2024 experience.
Patient Recruitment Management Platform
Our award-winning clinical trial recruitment management approach bridges the gap between patients and researchers. The platform allows patients to find relevant trials, understand trial information, and securely communicate with the study team. We announced the launch of our AI-powered clinical trial finder tool designed to simplify clinical trials with patient-friendly information, making them more efficient and inclusive.
Used by industry giants like Pfizer and Sanofi, leading academic medical centers, and patient advocacy groups, our platform was also recently selected to power the Fox Trial Finder for the Parkinson’s community.
Remote Data Collection Platform
Our remote data collection platform enables a wide range of participants, including astronauts, to engage in clinical research via mobile apps. In partnership with TRISH, we support health research data collection for several commercial human space missions. These projects aim to enhance healthcare delivery and data management for spaceflight participants, holding the potential to transform both space health and healthcare on Earth.
Our innovative work in the field of space health research has been prominently featured as – not one, but two groundbreaking papers focusing on decentralized clinical research in space missions – in the latest edition of Nature.
Clinical Research Songs for the Unsung: A Hit at DIA 2024
Our unique “Songs for the Unsung” offering was a resounding success. Over 100 visitors signed up to receive a personalized song, making our booth one of the most memorable spots at the conference. This creative engagement underscored our commitment to making connections and leaving a lasting impression.
Memorable Moments at #DIA2024
Our time at DIA 2024 was filled with impactful interactions and milestones.
We had fascinating discussions with representatives from Clario and Fortrea, learning about their AI models used in clinical trials.
A heartwarming highlight was assisting a caregiver in finding a suitable clinical trial for his mother, underscoring the real-world impact and importance of streamlined patient recruitment processes. We connected him to the research site live at our booth. This was a truly rewarding experience.
We reconnected with one of the earliest patient advocates Dave deBronkart in the clinical trials industry, reminiscing about our journey since the Google Health days in 2008. Dave is an inspiring international speaker on healthcare and health IT.
A Successful DIA 2024
Our DIA 2024 experience was a success, with a good number of visitors exploring our solutions and engaging with our team. The event provided a platform to showcase how our enterprise patient recruitment and remote data collection platforms are revolutionizing clinical trials, making processes more efficient and inclusive.
Thank you to everyone who visited us at Booth #1033 and participated in our “Songs for the Unsung” initiative. We look forward to continuing our journey of innovation and making a positive impact on clinical research. For more updates and to stay connected, follow us on social media. See you at #DIA2025 in Washington from June 15-19.