Connect up with TrialX at #Mobileclin2017 and #Dpharm2017 Conferences in Boston this September!
We are excited to participate and present at the 7th annual DPharm: Disruptive Innovations to Advance Clinical Trials (#Dpharm2017) conference in Boston in the first week of September 2017.
The first day of this conference, Sepetember 6th, 2017, is dedicated to discussions on Mobile in Clinical Trials (#Mobileclin2017).
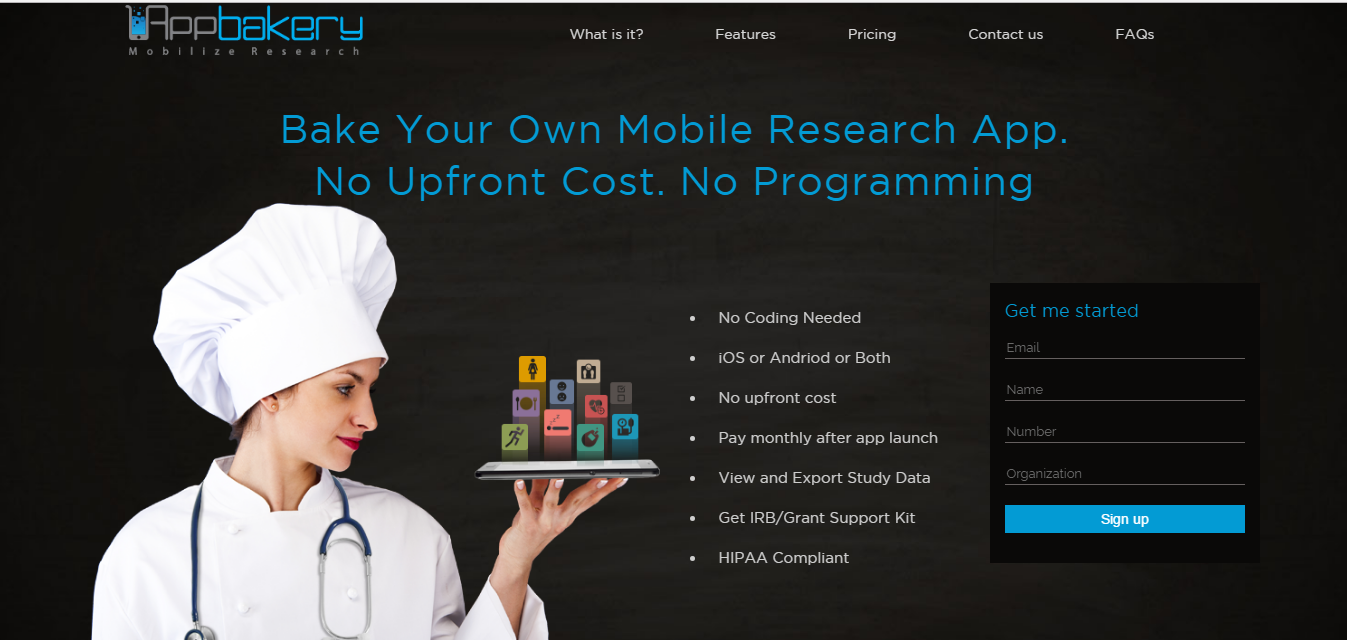
This year we will be sharing details of Appbakery – our DIY research study app building platform to collect patient data. We are also coming in with new enhanced features on iConnect – our online platform that enables patients to connect with clinical trial investigators, helping them reach their patient recruitment goals.
We hope to connect up with you in Boston, MA, between 6-8 September, 2017.
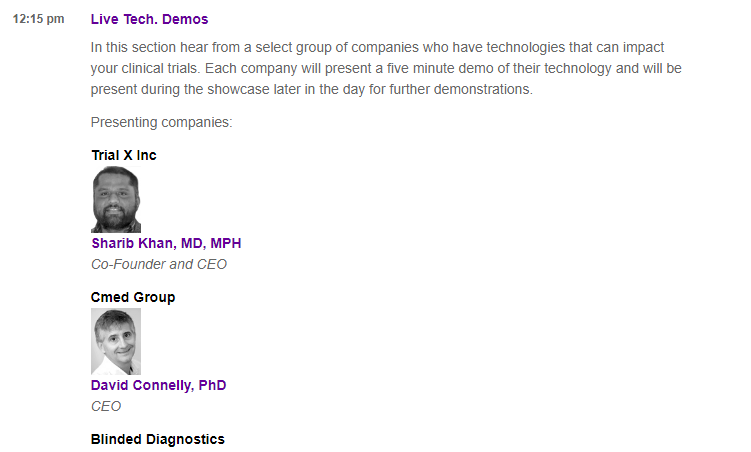
On the first day, at #mobileclin2017, TrialX co-founder and CEO, Dr. Sharib Khan will present a live demo of Appbakery, during the Live Tech demo session to be held at 12:15 PM. He will then be present during the Technology Showcase later in the day for further demos and questions.
The Appbakery platform allows researchers to design, create and deploy their entire mobile study app for their research studies, using a web interface, without needing to write any code.
The Mobile in Clinical Trials program will be chaired by Daniel Karlin of Pfizer, who will be leading a group of almost 20 speakers in discussing the importance, implementation, and impact of mobile in clinical research and clinical trials. #Mobileclin2017 will focus mainly on real-time implementation of mobile solutions, trials driving efficiency, ways to scale up, understand perceived barriers and ways to overcome them, moving past the hype of mhealth and producing evidence, optimizing the true value of mobile data, and more about Mobile 2.0, wearable, sensors, apps and mobile devices.
On 7-8 September at 3:40 PM, Dr. Khan will talk about ‘Transforming Patient Recruitment at the Enterprise Level with iConnect 2.0’ – our clinical trial management system currently being used by NYU, UPENN etc.
Led by GSK, Janssen and Pfizer, #Dpharm2017 will discuss case studies that demonstrate novel approaches to advancing clinical trials. Mobile Clinical Trials, Virtual Trials, Open Source Clinical Development are just a few examples of case studies featured. Each session aims to deliver a clear set of summary and key take-aways around challenges/opportunities, new business models, and innovations in science & technology applied to clinical development/trials.
This year’s DPharm 2017 has also announced a pre-conference workshop on Wednesday, September 6, 2017, 7-9 pm, where the attendees will have a chance to brainstorm and co-design an innovation to help patients in clinical trials. Barry Crist, Lead Investigator, Lilly Clinical Open Innovation Team, Eli Lilly & Company will lead the workshop.
Other activities at the conference include several networking sessions and DPharm Idol 2017 contest. The concluding portion of DPharm will be composed of a series of impactful sessions devoted to progress in innovation from all stakeholders.
We will be following and reporting about the happenings at the conference from our twitter account @TrialX. Please follow us and our tweets with hashtags #Mobileclin2017 and #DPharm2017 to stay informed!
Let’s connect up in Boston!