eConsent via Mobile Research Study Apps – An Improved way of gathering Informed Consent in Clinical Trials
Today we live in an era where we cannot underscore enough, the importance of informed consent in research and treatment – for the safety of patients as well as the physicians treating them.
Digital technology has changed the face of how people learn and communicate. Since the arrival of smartphones, use of mobile technology in clinical research has made the process of getting informed consent much easier. Gadgets like apps, mobile phones/smartphones, wearable technology, tablets, videos, interactive computers, all have the capacity to improve methods of informed consent.
Although still used widely, traditional paper based way of informed consent process is a tedious one, and inconvenient to both – the patient and the research team in many ways:
- The patient needs to be present at the research site.
- The patient has less time to go through, understand, and consult with family members. So, often times have to sign on the consent in a hurry.
- Patients do not have the leisure to review the consent at a later time if they want to be more informed.
- There is no way to supplement the consent form with additional info if required.
What’s Possible with e-consent?
Courtesy: mortgagecompliancemagazine.com
Using e-consent patients, clinical trial sites, and clinical trial investigators all have something to gain – just with few clicks and keystrokes.
Benefits to Patients
- Patients don’t have to be physically present at the research site.
- Patients can take time at their leisure to review, understand and discuss the consent form – so they don’t feel the pressure of answering right away.
- E-consent can be supplemented with pop-up definitions of difficult terms and interactive multi-media functions (audio, video), that are more engaging than traditional paper based method, thus help patients make more informed decisions. One great example of use of video in e-consent is the ADAPTABLE trial (Aspirin Dosing: A Patient-Centric Trial Assessing Benefits and Long-Term Effectiveness) — a very large pragmatic trial comparing two doses of aspirin.
Benefits to Investigators and Clinical Trial Sites
- Facilitates more interaction between participant and clinical trial site staff.
- Facilitates interaction between sponsors, ethics committees and clinical trial sites.
- One big advantage to the clinical trial site is easy storage and access of information. With all consent documents created, reviewed, modified and signed electronically, the site can store all the data at multiple locations for easy access.
- E-consent provides an opportunity to make clinical research more personalized and patient-centric.
What are Some of the Barriers to e-consent Adaptation?
- Lack of well-established processes.
- Concerns about security and confidentiality of data collected.
- Initial high expense for infrastructure to validate and mange electronic documents.
- Global acceptance of e-signatures.
Although some barriers still remain in the adoption of e-consent, the advantages seem to outweigh the concerns.
What is the Acceptability of e-consent Among Physicians?
The global clinical trial industry is ready for the change. Several randomized controlled trials comparing electronic vs paper-based consent process prove this. In a 2016 study, Warriner et al. compared the 2 methods for an osteoporosis study and concluded that the patients and office staff trended towards greater satisfaction with the tablet informed consent.
A risk-based survey done with UK physicians in 2016 shows that researchers are willing to use electronic consent processes, but most of them need some training. This survey also showed that e-consent process may be most appropriate for large epidemiology, observational or genetic studies or those studies which target hard to reach populations.
However, most of the studies agreed that we need further exploration of the overall effectiveness as well as cost-effectiveness of this method as recruitment strategy.
Last year in July, The Clinical Trials Transformation Initiative (CTTI) published the results of the project it convened to identify problems in the current informed consent process and to formulate recommendations for improvement. Based on this study, CTTI encourages researchers and sponsors to further explore the use of e-consent, by conducting interventions which demonstrate that e-consent processes:
- Help participants understand the content of the consent document better
- Help patients be more satisfied with their decision making
- Increase retention of study participants
- Increased protocol compliance by study participants
CTTI urges researchers to share the lessons learned from these studies, to achieve widespread e-consent adoption.
According to a literature review performed in this project, many participants already find the information on informed consent documents to be lengthy, complex and confusing. On the basis of expert interviews, a literature review, and multi-stakeholder meeting conducted to develop solutions for a more effective informed consent process, CTTI outlines some detailed examples of opportunities provided by e-consent that can be accessed in table 2 of the CTTI paper.
CTTI will present strategies & insights from its Mobile in Clinical Trials (MCT) program at #MobileClin2017 and #DPharm2017 conferences in Boston between 6-8 September.
Regulatory Requirements for use of e-consent in Clinical Trials
Many researchers don’t have complete knowledge about the regulatory requirements for implementing e-consent processes. While creating America Walks Study app we learned that the Text of the Informed Consent pages, when delivered via an app, is one of the most important documents for IRB approval of a research study. To avoid resubmissions and ensure compliance with regulations, e-consent forms should be validated with complete, accurate, legible, original, attributable, and consistent data with a full audit trail.
Another important regulatory requirement is that the app collecting e-consent should be HIPPA compliant. The information collected via e-consent forms such as a person’s name, age, and medical conditions etc. all come under Protected Health Information (PHI), which makes being HIPPA compliant mandatory, so as to ensure protection of patient’s/subject’s personal information. Moreover, if such information is to be shared with a healthcare provider, it must be protected by HIPPA laws. Read here about 5 most frequently encountered HIPAA hurdles and their solutions in building ResearchKit apps.
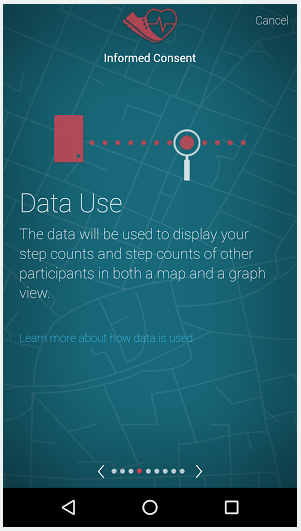
What does e-consent look like in ResearchKit Apps?
With the advent of ResearchKit in 2015, many leading research institutions such as Stanford, Rochester, and Johns Hopkins have used the technology to conduct research studies where they gathered informed consent and collected patient data remotely through smartphone apps. As a 2017 review article summarizes it succinctly “ResearchKit includes an e-consent process with a visual consent flow, composed of animated screens of consent elements, links to “learn more,” and a full consent form for review. In addition, the participant is given a screen to review and “opt-in” to allow access to each element of health or demographic data through the smartphone and a screen to choose whether the data can be shared with researchers worldwide.”
Last year at #mobileclin2016 we presented how we built a cross-platform research study app – America Walks Study – which allowed e-consent collection from study participants using both iOS and Android smartphones.
Here are some of the informed consent screens from the AWS app.
This year at #mobileclin2017 on September 6 in Boston, our CEO, Dr. Sharib Khan will demonstrate Appbakery – our DIY research study app building platform where researchers can build an app for their study in minutes, at a considerably low cost. Appbakery, may help researchers speed up several processes involved in clinical trials – such as – obtaining e-consent and creating surveys. Researchers can collect patient generated data remotely for a variety of clinical studies such as behavioral and cognitive studies, studies measuring medication adherence, studies measuring glucose monitoring in diabetes patients etc.
During #dpharm2017, Dr. Khan will also talk about transforming patient recruitment at the enterprise level with iConnect 2.0 – our patient recruitment management system.
If you are a researcher or physician looking to start a mobile based research study, and collect survey or sensor/wearables data using smartphone apps, you might want to learn more about Appbakery. Get in touch with us!