8 Key Things to Look for While Reading Clinical Trial Listings to Find a Matching Trial

Clinical trials are vital for advancing medical science and giving patients access to groundbreaking treatments. However, navigating clinical trial listings can often feel like an uphill battle, especially for patients and caregivers. While platforms like ClinicalTrials.gov provide extensive trial data, they are primarily designed for medical professionals, leaving non-experts struggling to identify relevant trials.
This disconnect creates several challenges:
- Complex Layouts: Listings are often dense, packed with text, and lack user-friendly navigation or filters.
- Technical Jargon: Terms such as “randomization,” “placebo,” and “endpoints” can be confusing for those without medical expertise.
- Unclear Eligibility Criteria: Many listings present requirements in technical formats, making it difficult for users to interpret and increasing the chances of missteps or misunderstandings.
Approximately 80% of clinical trials in the United States miss enrollment deadlines. A CISCRP 2023 Survey highlights that 12% of patients abandon their clinical trial search due to unclear or overly complex trial details.
If this process feels overwhelming, you’re not alone. This guide simplifies clinical trial listings, breaks down key terms, and provides a checklist to help you find relevant trials.
Basic Jargon You Need to Know Before Reading Clinical Trial Listings
- Principal Investigator (PI) – This is the lead researcher responsible for running the trial. The PI is usually a doctor or scientist who oversees all study aspects, including patient safety and data collection.
Example: If a trial is testing a new cancer drug at Johns Hopkins, the listing might say, “Principal Investigator: Dr. Jane Smith, MD, Oncology Researcher.”
- Eligibility Criteria – These are the rules that define who can or cannot participate in the study. They are usually divided into:
- Inclusion criteria: Conditions you must meet to join (e.g., “Participants must be between 18-65 years old and diagnosed with Type 2 diabetes”).
- Exclusion criteria: Conditions that disqualify you (e.g., “Participants who have had heart surgery in the past six months cannot enroll”).
- Intervention – This refers to the treatment, drug, procedure, or behavioral approach being tested. It could be a new medication, a surgical technique, or even a lifestyle program.
Example: In a weight-loss study, the intervention might be a new diet plan combined with an exercise routine.
- Phase: Describe the stage of the trial. For example, Phase I focuses on safety, while Phase III involves large-scale testing for effectiveness. Visit the FDA website to learn more about the four phases of clinical trials.
Example: If a new migraine drug is in Phase III, it means it has already been tested for safety and is now being compared to existing migraine treatments.
- Randomization – This is the process of assigning participants to different groups by chance to eliminate bias. Some people receive the experimental treatment, while others might get a standard treatment or a placebo.
Example: If you join a trial testing a new allergy medication, a computer might randomly assign you to either the drug group or the placebo group without you knowing which one you’re in.
- Placebo – A substance that looks like the real treatment but has no active ingredients. It helps researchers determine if the real treatment works better than no treatment at all.
Example: In a study for a new sleep aid, half of the participants might take the actual drug while the other half take a placebo to compare results.
- Endpoints – These are the specific goals the trial is measuring to determine success. Endpoints can be things like symptom improvement, survival rates, or fewer hospital visits.
Example: In a study for a new arthritis drug, an endpoint might be “a 50% reduction in joint pain after six months of treatment.”
By familiarizing yourself with these terms, you’ll be better equipped to understand trial listings and assess whether a study might be a good fit for you. For more details, you can explore the Novartis Glossary of Clinical Trial Terms.
Key Checklist for Reading Clinical Trial Listings
When reviewing a clinical trial listing, focus on these critical elements to ensure the trial meets your needs and expectations:
1. Trial Title and Objective
What to Look For: The trial’s title often summarizes its purpose. For example, a study titled “A Phase III Study of XYZ Drug in Treating Lung Cancer” tells you the trial is in an advanced stage and focuses on lung cancer. Additionally, the objective section outlines the specific aims of the trial, such as evaluating the drug’s effectiveness, safety, or impact on quality of life.
Why It Matters: Understanding both the title and objective helps determine if the trial aligns with your medical needs and treatment goals.
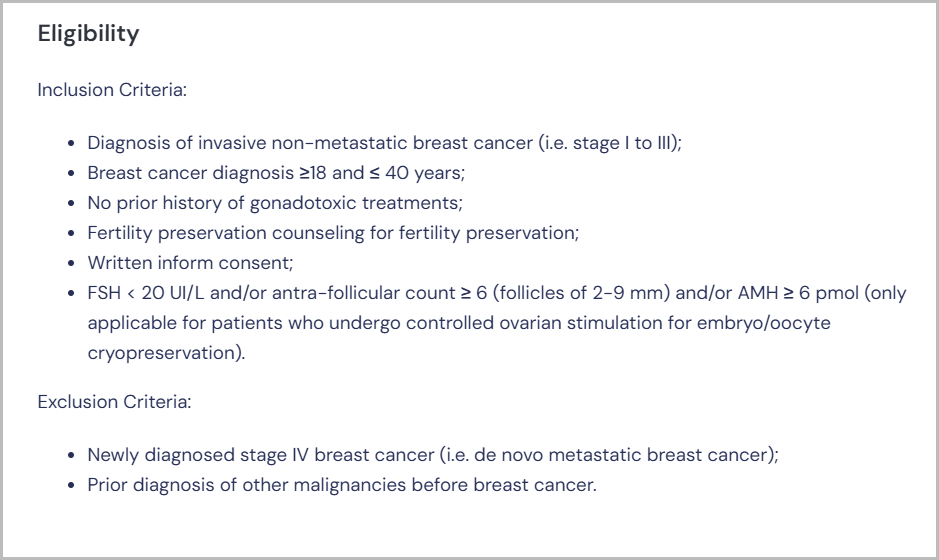
2. Eligibility Criteria
What to Look For: Carefully review the inclusion and exclusion criteria. These may specify age, gender, medical history, current health conditions, or prior treatments.
Why It Matters: Meeting the eligibility requirements is essential to participate. Misinterpretation could lead to wasted time or disqualification during screening.
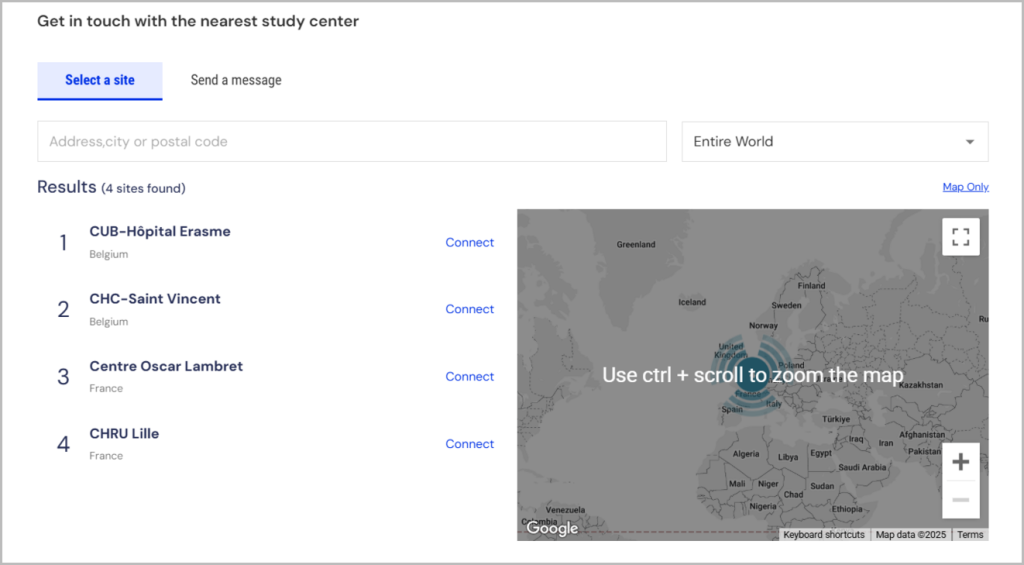
3. Study Location
What to Look For: The trial site’s address or whether remote participation is available. Some listings include multiple locations.
Why It Matters: Accessibility is a practical consideration, especially if regular visits are required. Remote options can significantly ease participation.
4. Intervention Details
What to Look For: The type of treatment (e.g., drug, device, therapy) and how it’s administered (e.g., oral, injection, surgery).
Why It Matters: Knowing the intervention type allows you to assess its relevance and feasibility for your condition and lifestyle.
5. Study Duration
What to Look For: The estimated length of the study, including the treatment period and follow-up.
Why It Matters: Ensure you’re prepared for the time commitment and any long-term requirements, such as periodic health checks.
6. Potential Risks and Benefits
What to Look For: Details about the potential risks and benefits associated with the trial, including possible side effects, complications, or improvements expected from the intervention.
Why It Matters: Though most clinical trials are relatively safe and follow strict ethical guidelines, understanding the risks helps participants make an informed decision. Knowing the potential benefits ensures the trial aligns with their health goals.
7. Expected results
What to Look For: The specific outcomes the trial is measuring, such as survival rates, symptom improvement, or disease remission.
Why It Matters: These endpoints will help you understand what the trial hopes to achieve and whether it aligns with your treatment goals.
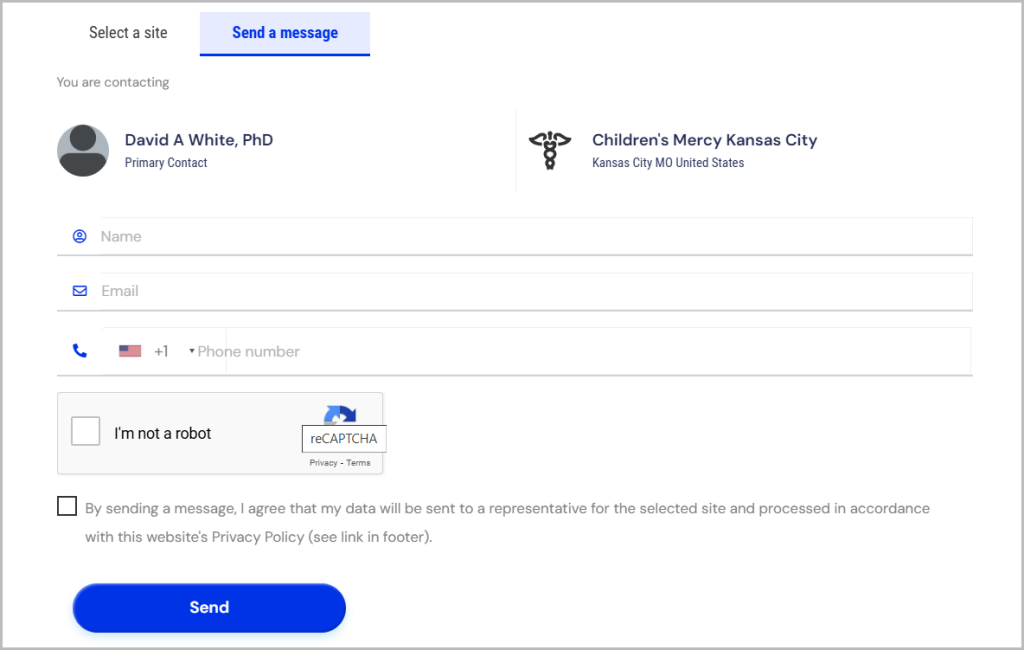
8. Contact Information and Funding Source
What to Look For: Ensure the trial listing includes the contact details of the trial coordinator or investigator and information about the funding source or sponsor (e.g., pharmaceutical company, government agency, or academic institution).
Why It Matters: Having a point of contact allows you to ask questions or clarify uncertainties before enrolling while knowing the sponsor provides insights into the trial’s credibility, focus, and available resources.
Additional Information To Consider While Reading A Clinical Trial Listing
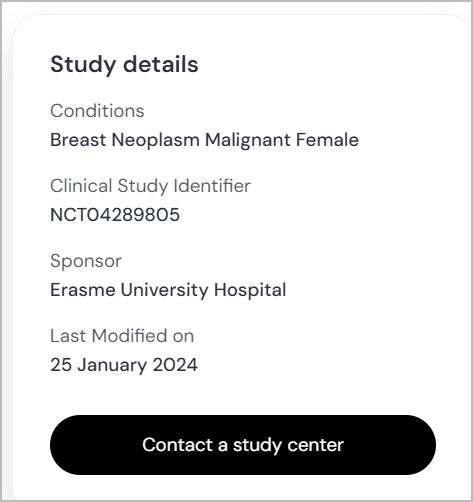
- NCT Number
What to Look For: Every registered clinical trial has a unique NCT (National Clinical Trial) number (e.g., NCT01234567), assigned by ClinicalTrials.gov.
Why It Matters: This number allows you to verify trial details on official registries, ensuring accuracy and legitimacy. It also helps you track trial updates or share information with your doctor.
- Trial Phase
What to Look For: Check the trial phase to understand its stage in development.
Why It Matters: Later-phase trials have more data on effectiveness, while early-phase trials focus on initial testing.
Why Use TrialX to Simplify Your Search
TrialX is built to make it easier and faster to find matching clinical trials with its innovative AI-powered tools. Here’s how.
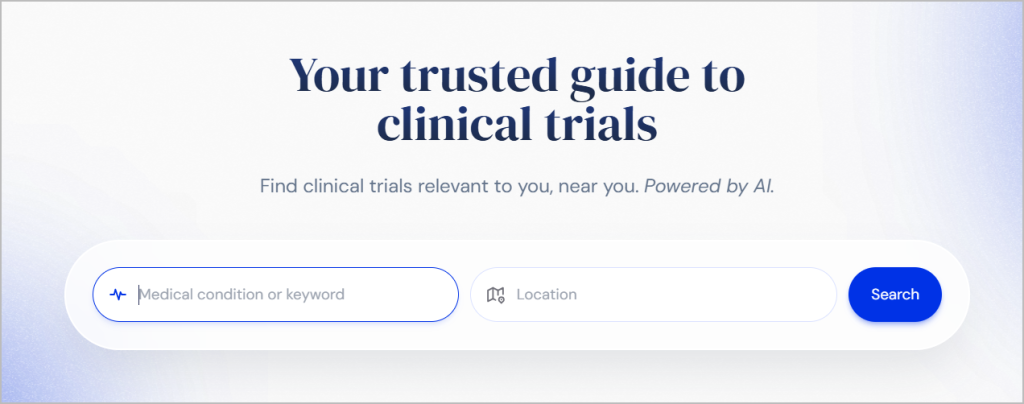
Easy Search Options
Search for trials using simple keywords, such as medical conditions or locations. TrialX’s user-friendly interface eliminates the need for extensive technical knowledge.
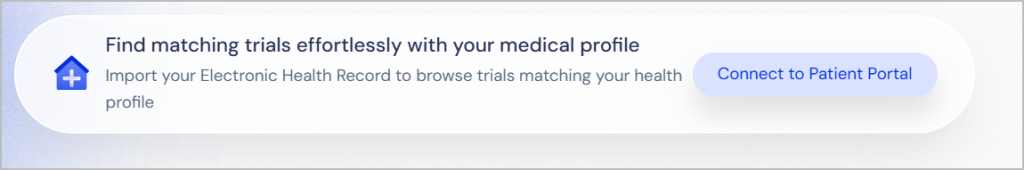
Electronic Health Record (EHR) Integration
Import your health data via your Electronic Health Record (EHR) to find trials tailored to your specific medical history.
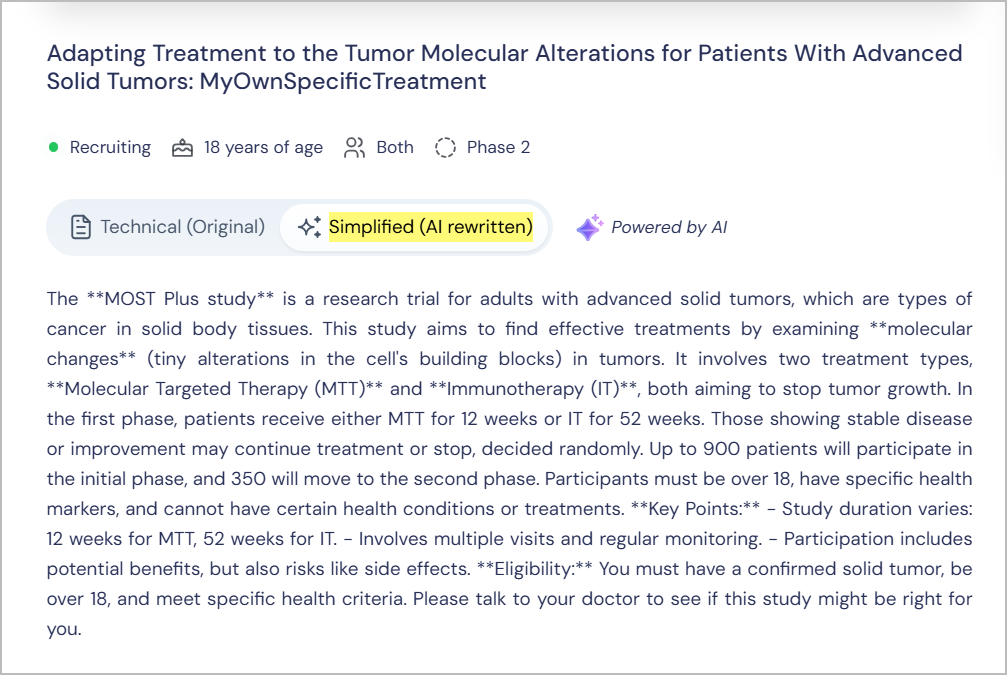
AI-Powered Jargon Simplification
TrialX uses AI tools to translate complex clinical jargon into plain language, ensuring that you fully understand trial details before making decisions.
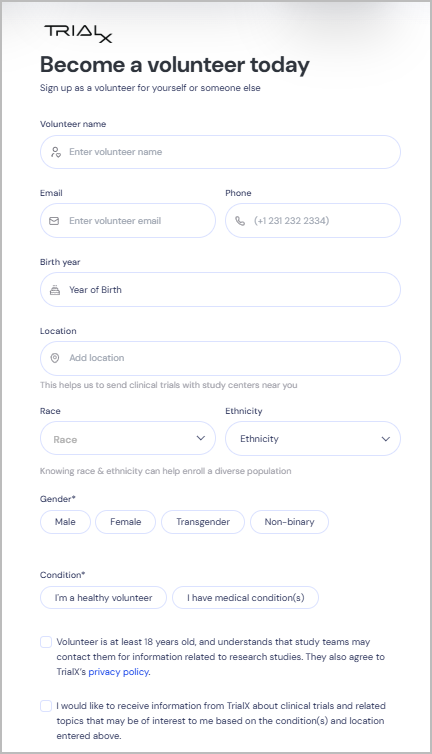
Volunteer Registry
Join the TrialX volunteer registry to receive updates on relevant trials and increase your chances of finding opportunities that fit your health profile.
Finding the right clinical trial may seem challenging at first, but with the right tools and understanding, it becomes much more manageable. Empower yourself by learning the key terms, using a structured approach to evaluate trial listings, and seeking out resources designed with patients in mind.
Whether you’re looking for trials based on your condition, location, or medical history, TrialX offers the support and tools you need to find the best fit for your needs.
Visit TrialX to discover how we can help you connect to the right clinical trial.