4 Patient-Centric Approaches by Which TrialX is Enhancing Clinical Trial Participation

The clinical trial industry has undergone a radical transformation over the last decade. Historically, trial participants were often seen as mere data points, rather than being considered as active collaborators in research. Many trials were designed to address key scientific questions but failed to consider the patient experience, leading to high dropout rates, recruitment challenges, and data inconsistencies.
Today, this approach is rapidly changing. Patients are now more informed than ever, thanks to technology, social media, and easy access to medical information. This shift has led to increased demands for transparency, ease of participation, and better trial experiences.
At the recently held 12th Annual Patients as Partners Conference, a leading event dedicated to amplifying the patient voice in clinical research, industry leaders highlighted the importance of prioritizing the needs, preferences, and well-being of participants, not just as an ethical choice but also a strategic advantage. In one of the panel discussions – Enhancing Patient Engagement Through Artificial Intelligence: Insights and Experience from the Field, -our CEO and co-founder, Sharib Khan, shared how our AI-driven solutions are revolutionizing patient-centricity by facilitating trial accessibility for a diverse population and making participation more engaging with simplified clinical trial language and conversational AI navigators to guide participants.
In this blog, we will explore what patient-centricity in clinical trials really means, why it matters, and how TrialX is leading the way in making clinical trials more accessible and patient-friendly.
What is a Patient-Centric Approach in Clinical Trials?
A patient-centric clinical trial is one that places patients at the center of the research process. Instead of being passive participants, patients are treated as partners, with their needs, expectations, and convenience taken into account. This approach focuses on making trials more accessible, understandable, and less burdensome, ultimately improving patient recruitment, retention, and data quality.
Key Milestones in the Shift to Patient-Centricity
In 2012, the U.S. Food and Drug Administration (FDA) launched the Patient-Focused Drug Development (PFDD) Initiative as part of the Prescription Drug User Fee Act (PDUFA V). This initiative aimed to systematically gather patient perspectives on specific diseases and their treatments (FDA PFDD Initiative).
PFDD meetings are designed to engage patients directly, eliciting their insights on two key areas:
- The most significant symptoms of their condition and how it impacts their daily life.
- Their current approaches to treatment, including what they find beneficial or inadequate.
Between 2012 and 2017, under PDUFA V, the FDA conducted 24 disease-specific PFDD meetings. These meetings brought together key stakeholders—including patient advocates, researchers, drug developers, and healthcare providers—to hear directly from patients about the challenges they face. A key output of these discussions is the ‘Voice of the Patient’ reports, summarizing patient perspectives and informing future drug development
In 2017, the European Medicines Agency (EMA) released a comprehensive report on patient and consumer involvement in regulatory activities. This report emphasized that patient input should not just be collected but should actively shape clinical trial protocols, regulatory evaluations, and drug approvals. The EMA outlined formal mechanisms for integrating patient perspectives into the drug development process, ensuring that new treatments better reflect real-world patient needs.
The COVID-19 pandemic forced the clinical trial industry to innovate rapidly and adopt decentralized methods, making participation more accessible and patient-friendly. Key changes included:
- Remote patient monitoring using wearable devices and telemedicine.
- Digital pre-screening and eConsent processes to reduce site visits.
- Home-based sample collection and virtual consultations.
Decentralized trials made it easier for diverse patient populations to participate, reducing geographical and logistical barriers. Post-pandemic, these innovations have become standard practice, accelerating the industry’s shift toward patient-centricity.
How is TrialX Enhancing Clinical Trial Participation?
Here are 4 patient-centric approaches by which we are helping prospective clinical trial participants find and participate in relevant clinical trials.
1. Clinical Trial Finder Tool – Connecting Patients to the Right Trials
Finding a clinical trial that meets a patient’s specific health condition, location, and eligibility criteria can be daunting. Many patients struggle with platforms like ClinicalTrials.gov, which often present overwhelming, jargon-heavy trial descriptions and unstructured listings. Our TrialX Clinical Trial Finder Tool simplifies this process by offering a personalized, easy-to-navigate solution.
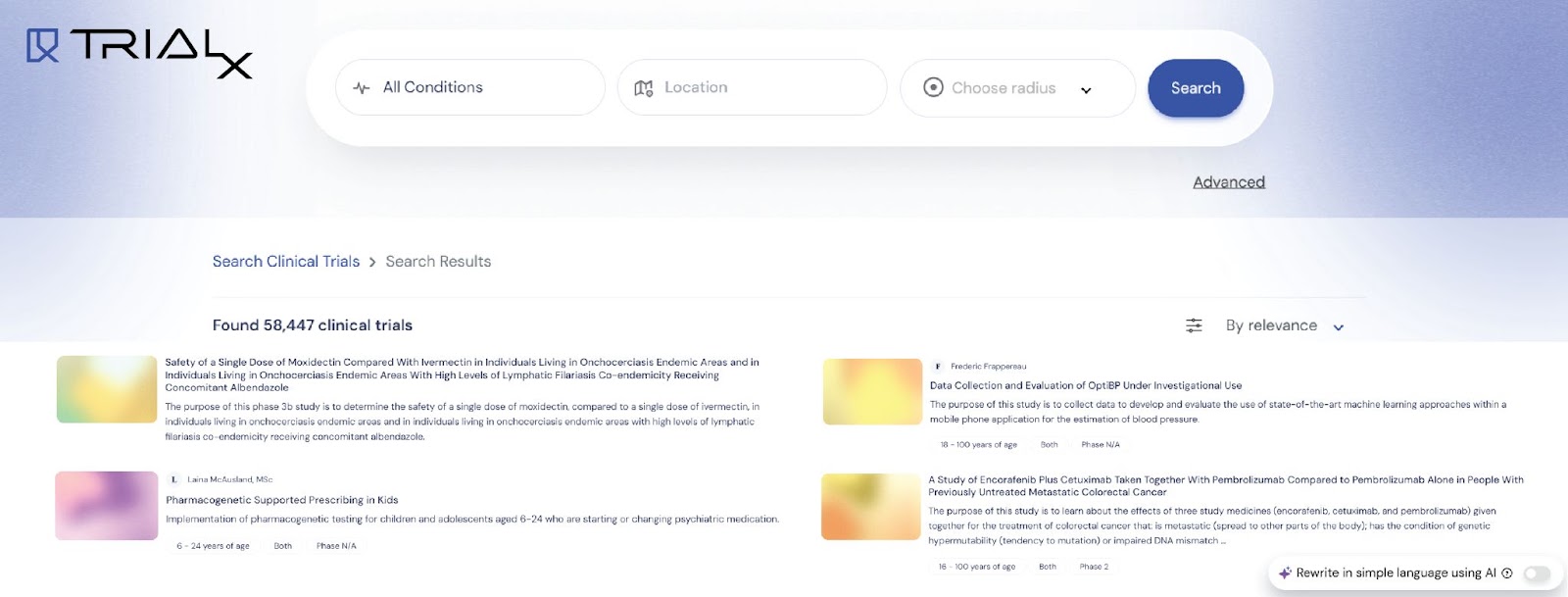
Key Features:
- Customized trial search based on health conditions and locations.
- Seamless EHR integration to refine searches and deliver the most accurate trial matches.
- Advanced user-friendly interface filters trials by location, phase, study type, gender, age group, and sponsor.
- Guided search provides a step-by-step overview of clinical trials, including a “Clinical Trial Primer” to educate patients on benefits, risks, and participation steps.
- Smart eligibility matching uses AI-driven filters to match patients with trials that align with their medical conditions and eligibility criteria, eliminating irrelevant listings.
- Direct contact options with “Call Now” and “Send Email” buttons, along with pre-written templates, help patients easily connect with study teams.
Top research institutions, including Penn Medicine, Indiana CTSI, and NYU Langone Health, have integrated our tool to streamline patient recruitment. We are supporting leading advocacy groups such as the Michael J. Fox Foundation, Sickle Cell Disease Association of America, Let’s Win Pancreatic Cancer, and the Reflections Initiative, helping patients access crucial clinical trial opportunities with our AI-powered clinical trial finders.
Explore more: https://trialx.com
2. Simplifying Clinical Trial Information using AI & & Providing Multilingual Support
Many trials use complex terms that are difficult for non-specialists to interpret, leading to confusion and lower engagement.
How our AI-based solutions help:
- Our AI-based solutions convert complex trial descriptions into clear, patient-friendly language, making it easier to understand eligibility and procedures.
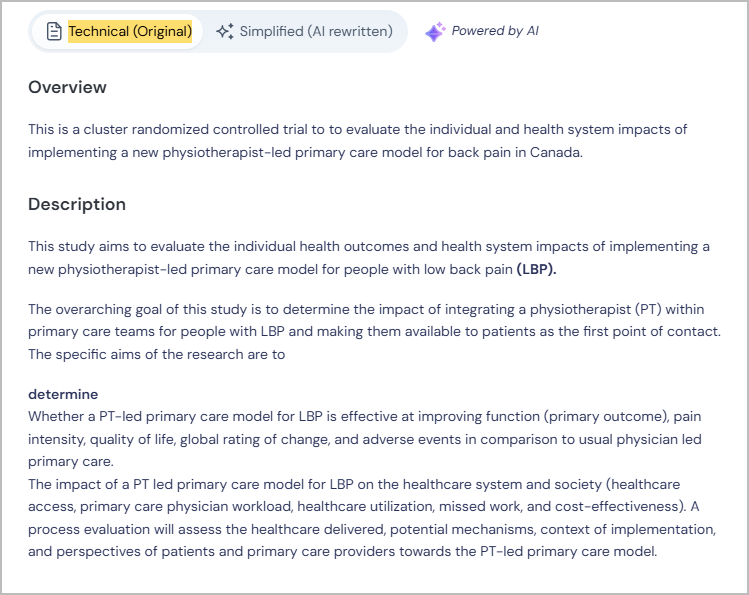
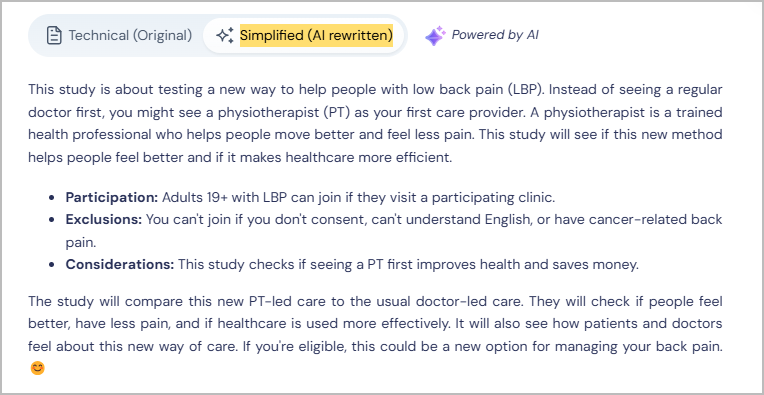
- Provides multilingual support, automatically translating trial details into multiple languages, expanding access to non-English speakers.
These features ensure that patients feel confident and informed before making decisions about participation.
3. Facilitating clinical trial participation with remote data collection
TrialX is transforming remote data collection with mobile apps that enable seamless electronic data capture and symptom tracking—reducing the burden on participants and improving data quality.
Key Benefits of Remote Data Collection in Clinical Trials
- Automated reminders for surveys, medication intake, and symptom tracking help participants stay engaged, improving compliance and reducing missed data points.
- Participants can conveniently submit data from home, significantly reducing the need for frequent site visits and lowering the burden of travel.
- Researchers gain real-time access to patient-reported outcomes, allowing for faster data analysis, trend identification, and timely decision-making.
- Digital data collection minimizes errors caused by manual entry, ensuring greater accuracy and reliability in trial data.
- Remote access enables participation from diverse geographic locations, making trials more inclusive and representative of broader populations.
- Secure data collection with compliance with regulations such as HIPAA and GDPR, ensuring patient confidentiality.
- Integration with wearables and apps to enable continuous, real-time data collection, providing deeper insights into patient health trends.
TrialX’s remote data collection solutions are transforming clinical research by enabling seamless participation both on Earth and in space. Beyond supporting ongoing clinical studies, our platform facilitates the collection of space health research data for missions such as Inspiration4, Axiom 1, Axiom 2, Axiom 3, and Polaris Dawn.
We recently partnered with the University of Iowa and UCLA to develop the SAYSTOP Study App, designed for a study on antibiotic resistance in UTI treatment. The app allows participants to track antibiotic use, report symptoms remotely, and receive automated reminders. By leveraging decentralized trial technology, TrialX is helping researchers improve data collection, expand patient access, and accelerate clinical research breakthroughs.
4. Volunteer Registry – Building a Community of Trial-Ready Participants
Recruiting the right participants remains one of the most challenging aspects of clinical research. At TrialX, we have built a Volunteer Registry to help patients pre-register for trials that match their health profile and interests.
How It Works:
- Patients can sign up, enter basic health information, and receive alerts when a relevant trial is available.
- Sponsors and researchers can search a database of registered participants based on specific criteria and connect with potential candidates, making recruitment more efficient.
- Participants stay informed about upcoming research opportunities without having to search repeatedly.
Why This Matters:
Up to 85% of clinical trials fail to recruit or retain enough participants, leading to delays and increased costs despite nearly $1.9 billion spent annually on recruitment. Our Volunteer Registry streamlines this process by proactively connecting eligible participants with relevant trials, reducing recruitment failures, and accelerating enrollment for faster medical advancements.
Join our volunteer registry here.
Building a Patient-First Future in Clinical Research
For too long, clinical trials have prioritized data collection while overlooking the challenges patients face in participating. Complex eligibility criteria and cumbersome processes create unnecessary barriers, discouraging many potential volunteers. But successful trials don’t just need participants—they need engaged, informed, and willing partners.
Sponsors who prioritize ease, transparency, and accessibility can enhance recruitment, improve retention, and generate higher-quality data. TrialX is leading this shift with AI-driven matching, digital pre-screening, and remote data collection, making trials more seamless and patient-friendly. These innovations reduce participant burden, streamline enrollment, and accelerate medical advancements.
The future of clinical research depends on collaboration between sponsors, technology providers, and patients. By embracing patient-first strategies, we can build a more inclusive, efficient, and impactful clinical trial ecosystem.
Learn more about our solutions at TrialX.com.



