Top 4 Reasons Why Patients Don’t Take Part in Clinical Trials
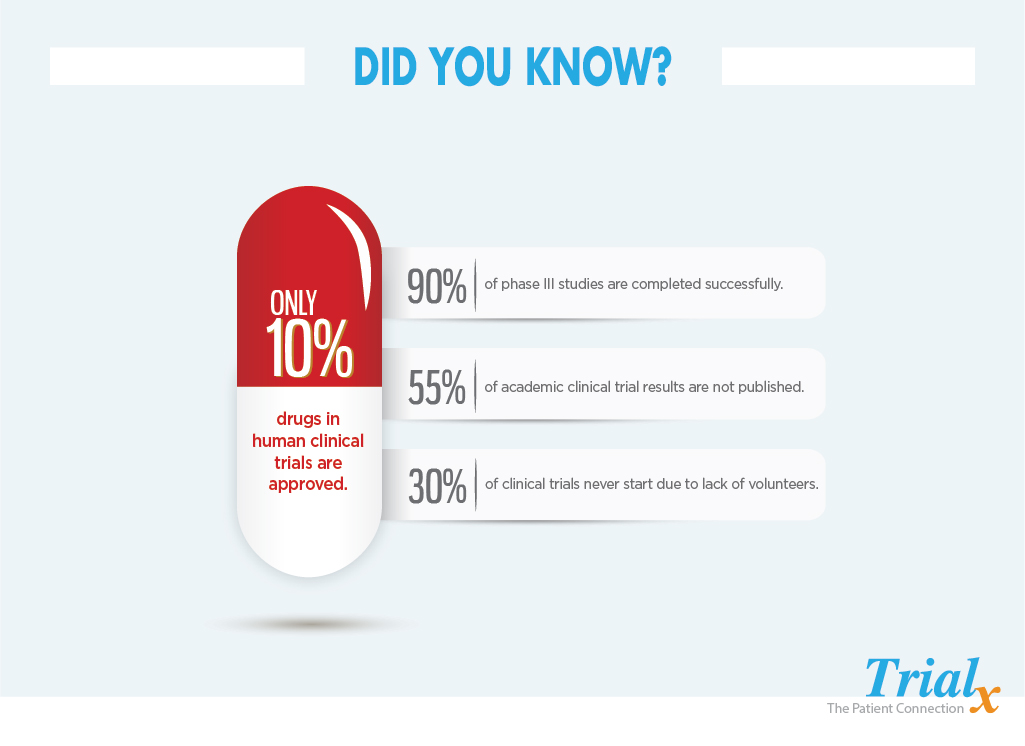
Only 10% of all drugs in human clinical trials become an approved drug. Nevertheless, clinical trials are imperative for discovering novel treatments to ever-changing diseases, and patients are an integral part of it. Sadly though, 30% of clinical trials never even get off the ground because of a lack of volunteers and 20% of cancer clinical trials never finish due to poor accrual.
Why is human testing important?
Looking at the poor state of clinical trials, you might want to ask, if it’s that costly and risky, why do human trials at all? Aren’t there other ways out to test a drug?
Well, human and animal systems are different. Humans share only 90% of their DNA with mice and 98.8% with chimpanzees. Although in their initial stages of development, drugs are tested on animals (preclinical testing) to show that they are working, unless a new drug is tested on patients, we cannot know how the drug is interacting within the human system, what is its efficacy and what are its adverse effects.
There are some other methods that are being explored right now. For example, the testing of drugs on cultured human cells and tissues grown invitro. However, these methods are being developed to replace animal testing mainly and not human trials. These methods themselves are still in their infancy and may take a while to be approved for replacing animal testing. So it may take some time for these or any other method to be approved to replace human testing. Until then, no matter how difficult an endeavor, human trials remain the gold standard for testing and approval of novel drugs.
How are clinical trials beneficial for the patients?
Many patients are not aware about clinical trials at all, others lack information on available trials, and most of them don’t know how to get involved in one. Further, many patients are not aware of the benefits of enrolling in a clinical trial.
By taking part in clinical trials, patients not only help other patients by facilitating drug development, but may also save several lives including their own. Clinical trials allow patients to get access to newest drugs and therapies, often times at a cost that is more affordable compared to when they have to purchase the drug. Moreover, they get the highest level of expertise and care with a new therapy that complements the current standard of treatment.
Donna Fernandez a big proponent of clinical trials urges fellow patients to participate in a trial and says,
Join a clinical trial! Who knows? The life you save may be your own!!
What are the 4 big reasons that scare patients away from Clinical Trials?
Lack of awareness of clinical trials may be a big reason for lesser patient participation, but what about those who are aware and still choose not to participate? A survey by Harris Interactive, Inc., showed that of the patients who knew about clinical trials, 71% chose not to participate. So, what are the reasons that pull these patients away from clinical trials?
Here are the some of the most important ones to know:
1) Uncertainty of treatment – Fear of being treated as a guinea pig and getting a placebo is the most common factor that pulls patients away from clinical trials. However, it is important for patients to understand that clinical trials usually run parallel to their regular clinical care.
Most clinical trials are randomized to put patients in the experimental arm or the control arm, where the control arm is supposed to be on a placebo. However, in the case of cancer trials, placebo is never used in lieu of standard treatment. Instead, the standard of care chemotherapy is given to the control arm in place of a placebo.
Moreover, most of the times the trials are designed such that the patients would be allowed to cross over to the experimental drug, in case their disease continue to progress.
2) Uncertainty of results/adverse effects – People are afraid whether the new therapy will work to improve their health or worsen their disease. However, unlike old times, currently participant’s right are protected by regulatory bodies like the Institutional Review Board (IRB), and the trials are designed to minimize any adverse effects to participants.
3) Uncertainty of resources needed to take part in a clinical trial – Cost is a big factor for patients while considering to get involved in a trial. Many studies may ask patients to partially pay for the new therapy or tests involved, but most of the time treatments are free for participants.
Some trials even pay the participants for enrolling in a trial. However, FDA suggests people must learn thoroughly about the risks involved in a study before enrolling, and not get lured by the free treatment or money being offered.
Other than cost, many patients may not be sure of the time commitment they would need to visit trial sites for repeated assessments. Most trials require patients to visit multiple times and each visit may be 5-6 hours long.
4) Long travel – When you are sick you don’t want to travel far off from your home, because that is the most convenient place that can be. Many patients fear that taking part in clinical trials means travelling to far off locations. Moreover, uncertainty of number of repeated visits they may have to make to the clinic pulls them away from getting involved. Lately, technology has helped patients overcome some of this fear by offering several clinical trial finders. Clinical trial finders like Dory can help patients connect to clinical trials investigators near their place, so as to avoid long travels.
At the recently held HIMSS 2017 conference , international patient engagement activist, speaker and author Dave deBronkart said “Knowledge is power” and “Healthcare truly is all about knowledge”.
Think about it. Would you ever try to buy a new phone or a furniture without learning and understanding how it’s going to solve your current requirements and if it is worth the price? Here, we are talking about people with life threatening diseases, and we are asking them to try a new experimental drug. So, we got to educate them and involve them in the decision making process.
Talking about improving our current clinical trial processes, TJ Sharpe, a stage 4 melanoma survivor and patient advocate says,
Having the patient voice involved much earlier in the process has a lot of return on investment. It’s not a very expensive thing to do, and it provides some invaluable feedback.
The stakeholders of the research and drug development industry must understand the fears patients harbor, and work toward educating more and more patients to clear their misconceptions, so as to build up an environment where the patients have easy access to updated information and feel more involved in their treatment decisions right from the beginning.
We do our bit in educating and helping patients find relevant clinical trials conveniently through our clinical trial finder tools like Dory. Dory asks a series of questions about patient’s medical condition and their location, so as to find the most suitable study that is available at a research center/hospital nearest to them.
You can try Dory tool here and read more about it here .